Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1)
- PMID: 21262968
- PMCID: PMC3060549
- DOI: 10.1074/jbc.M110.185983
Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1)
Abstract
Latent membrane protein 1 (LMP1), encoded by Epstein-Barr virus, is required for EBV-mediated B cell transformation and plays a significant role in the development of posttransplant B cell lymphomas. LMP1 has also been implicated in exacerbation of autoimmune diseases such as systemic lupus erythematosus. LMP1 is a constitutively active functional mimic of the tumor necrosis factor receptor superfamily member CD40, utilizing tumor necrosis factor receptor-associated factor (TRAF) adaptor proteins to induce signaling. However, LMP1-mediated B cell activation is amplified and sustained compared with CD40. We have previously shown that LMP1 and CD40 use TRAFs 1, 2, 3, and 5 differently. TRAF6 is important for CD40 signaling, but the role of TRAF6 in LMP1 signaling in B cells is not clear. Although TRAF6 binds directly to CD40, TRAF6 interaction with LMP1 in B cells has not been characterized. Here we tested the hypothesis that TRAF6 is a critical regulator of LMP1 signaling in B cells, either as part of a receptor-associated complex and/or as a cytoplasmic adaptor protein. Using TRAF6-deficient B cells, we determined that TRAF6 was critical for LMP1-mediated B cell activation. Although CD40-mediated TRAF6-dependent signaling does not require the TRAF6 receptor-binding domain, we found that LMP1 signaling required the presence of this domain. Furthermore, TRAF6 was recruited to the LMP1 signaling complex via the TRAF1/2/3/5 binding site within the cytoplasmic domain of LMP1.
Figures


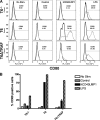
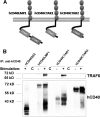
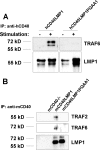
Similar articles
-
TRAF6 is a critical regulator of LMP1 functions in vivo.Int Immunol. 2014 Mar;26(3):149-58. doi: 10.1093/intimm/dxt052. Epub 2013 Oct 29. Int Immunol. 2014. PMID: 24170780 Free PMC article.
-
Roles of the kinase TAK1 in TRAF6-dependent signaling by CD40 and its oncogenic viral mimic, LMP1.PLoS One. 2012;7(7):e42478. doi: 10.1371/journal.pone.0042478. Epub 2012 Jul 30. PLoS One. 2012. PMID: 22860133 Free PMC article.
-
TRAF binding is required for a distinct subset of in vivo B cell functions of the oncoprotein LMP1.J Immunol. 2012 Dec 1;189(11):5165-70. doi: 10.4049/jimmunol.1201821. Epub 2012 Oct 29. J Immunol. 2012. PMID: 23109728 Free PMC article.
-
[The TRAF family protein-mediated B cell proliferation signal and the mechanism of LMP1-induced B cell transformation].Nihon Rinsho. 1997 Feb;55(2):299-304. Nihon Rinsho. 1997. PMID: 9046814 Review. Japanese.
-
Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1.Immunol Rev. 2010 Sep;237(1):226-48. doi: 10.1111/j.1600-065X.2010.00932.x. Immunol Rev. 2010. PMID: 20727039 Review.
Cited by
-
Exosomal sorting of the viral oncoprotein LMP1 is restrained by TRAF2 association at signalling endosomes.J Extracell Vesicles. 2015 Apr 10;4:26334. doi: 10.3402/jev.v4.26334. eCollection 2015. J Extracell Vesicles. 2015. PMID: 25865256 Free PMC article.
-
Lupus and Epstein-Barr.Curr Opin Rheumatol. 2012 Jul;24(4):383-8. doi: 10.1097/BOR.0b013e3283535801. Curr Opin Rheumatol. 2012. PMID: 22504579 Free PMC article. Review.
-
Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-κB signaling during productive replication.J Virol. 2013 Apr;87(7):4060-70. doi: 10.1128/JVI.02020-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365429 Free PMC article.
-
TRAF6 is a critical regulator of LMP1 functions in vivo.Int Immunol. 2014 Mar;26(3):149-58. doi: 10.1093/intimm/dxt052. Epub 2013 Oct 29. Int Immunol. 2014. PMID: 24170780 Free PMC article.
-
Comparison of the peptide binding preferences of three closely related TRAF paralogs: TRAF2, TRAF3, and TRAF5.Protein Sci. 2016 Jul;25(7):1273-89. doi: 10.1002/pro.2881. Epub 2016 Feb 3. Protein Sci. 2016. PMID: 26779844 Free PMC article.
References
-
- Pai S., Khanna R. (2001) Semin. Cancer Biol. 11, 455–460 - PubMed
-
- Küppers R. (2003) Nat. Rev. Immunol. 3, 801–812 - PubMed
-
- Busch L. K., Bishop G. A. (1999) J. Immunol. 162, 2555–2561 - PubMed
-
- Schubert S., Abdul-Khaliq H., Lehmkuhl H. B., Yegitbasi M., Reinke P., Kebelmann-Betzig C., Hauptmann K., Gross-Wieltsch U., Hetzer R., Berger F. (2009) Pediatr. Transplant 13, 54–62 - PubMed
-
- Schubert S., Renner C., Hammer M., Abdul-Khaliq H., Lehmkuhl H. B., Berger F., Hetzer R., Reinke P. (2008) J. Heart Lung Transplant. 27, 100–105 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

