Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis
- PMID: 21262973
- PMCID: PMC3069416
- DOI: 10.1074/jbc.M110.154625
Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis
Abstract
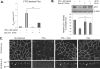
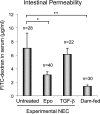
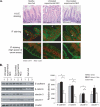
The impermeant nature of the intestinal barrier is maintained by tight junctions (TJs) formed between adjacent intestinal epithelial cells. Disruption of TJs and loss of barrier function are associated with a number of gastrointestinal diseases, including neonatal necrotizing enterocolitis (NEC), the leading cause of death from gastrointestinal diseases in preterm infants. Human milk is protective against NEC, and the human milk factor erythropoietin (Epo) has been shown to protect endothelial cell-cell and blood-brain barriers. We hypothesized that Epo may also protect intestinal epithelial barriers, thereby lowering the incidence of NEC. Our data demonstrate that Epo protects enterocyte barrier function by supporting expression of the TJ protein ZO-1. As immaturity is a key factor in NEC, Epo regulation of ZO-1 in the human fetal immature H4 intestinal epithelial cell line was examined and demonstrated Epo-stimulated ZO-1 expression in a dose-dependent manner through the PI3K/Akt pathway. In a rat NEC model, oral administration of Epo lowered the incidence of NEC from 45 to 23% with statistical significance. In addition, Epo treatment protected intestinal barrier function and prevented loss of ZO-1 at the TJs in vivo. These effects were associated with elevated Akt phosphorylation in the intestine. This study reveals a novel role of Epo in the regulation of intestinal epithelial TJs and barrier function and suggests the possible use of enteral Epo as a therapeutic agent for gut diseases.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

