A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice
- PMID: 21263114
- PMCID: PMC3621110
- DOI: 10.1161/HYPERTENSIONAHA.110.163329
A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice
Abstract
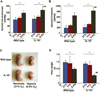
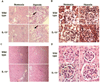
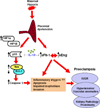
Hypoxia has been implicated in the pathogenesis of preeclampsia, a hypertensive disorder of pregnancy. However, in vivo evidence and mechanistic understanding remain elusive. Preeclampsia is associated with impaired placental angiogenesis. We have recently shown that interleukin (IL)-10 can support trophoblast-driven endovascular crosstalk. Accordingly, we hypothesize that pathological levels of oxygen coupled with IL-10 deficiency induce severe preeclampsia-like features coupled with elevated production of antiangiogenic factors, apoptotic pathways, and placental injury. Exposure of pregnant wild-type and IL-10(-/-) mice to 9.5% oxygen resulted in graded placental injury and systemic symptoms of renal pathology, proteinuria (wild-type 645.15 ± 115.73 versus 198.09 ± 93.45; IL-10(-/-) 819.31 ± 127.85 versus 221.45 ± 82.73 μg/mg/24 hours) and hypertension (wild-type 118.37 ± 14.45 versus 78.67 ± 14.07; IL-10(-/-) 136.03 ± 22.59 versus 83.97 ± 18.25 mm Hg). Recombinant IL-10 reversed hypoxia-induced features in pregnant IL-10(-/-) mice confirming the protective role of IL-10 in preeclampsia. Hypoxic exposure caused marked elevation of soluble fms-like tyrosine kinase 1 (110.8 ± 20.1 versus 44.7 ± 11.9 ng/mL) in IL-10(-/-) mice compared with their wild-type counterparts (81.6 ± 13.1 versus 41.2 ± 8.9 ng/mL), whereas soluble endoglin was induced to similar levels in both strains (approximately 380 ± 50 versus 180 ± 31 ng/mL). Hypoxia-induced elevation of p53 was associated with marked induction of proapoptotic protein Bax, downregulation of Bcl-2, and trophoblast-specific apoptosis in utero-placental tissue. Collectively, we conclude that severe preeclampsia pathology could be triggered under certain threshold oxygen levels coupled with intrinsic IL-10 deficiency, which lead to excessive activation of antiangiogenic and apoptotic pathways.
Figures
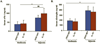




Similar articles
-
Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats.Hypertension. 2013 Sep;62(3):599-607. doi: 10.1161/HYPERTENSIONAHA.113.01449. Epub 2013 Jul 1. Hypertension. 2013. PMID: 23817493 Free PMC article.
-
Activating Transcription Factor 3 Is Reduced in Preeclamptic Placentas and Negatively Regulates sFlt-1 (Soluble fms-Like Tyrosine Kinase 1), Soluble Endoglin, and Proinflammatory Cytokines in Placenta.Hypertension. 2017 Nov;70(5):1014-1024. doi: 10.1161/HYPERTENSIONAHA.117.09548. Epub 2017 Sep 25. Hypertension. 2017. PMID: 28947613
-
Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia.Hypertension. 2014 Mar;63(3):595-606. doi: 10.1161/HYPERTENSIONAHA.113.02458. Epub 2013 Dec 9. Hypertension. 2014. PMID: 24324043 Free PMC article.
-
Aetiology and physiopathology of preeclampsia and related forms.Acta Clin Belg. 2010 Jul-Aug;65(4):237-41. doi: 10.1179/acb.2010.051. Acta Clin Belg. 2010. PMID: 20954461 Review.
-
Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia.Am J Physiol Heart Circ Physiol. 2020 Sep 1;319(3):H661-H681. doi: 10.1152/ajpheart.00202.2020. Epub 2020 Aug 7. Am J Physiol Heart Circ Physiol. 2020. PMID: 32762557 Free PMC article. Review.
Cited by
-
Preeclampsia is a biomarker for vascular disease in both mother and child: the need for a medical alert system.Int J Pediatr. 2013;2013:953150. doi: 10.1155/2013/953150. Epub 2013 Apr 16. Int J Pediatr. 2013. PMID: 23690796 Free PMC article.
-
IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia.Hypertens Pregnancy. 2015;34(3):291-306. doi: 10.3109/10641955.2015.1032054. Epub 2015 May 21. Hypertens Pregnancy. 2015. PMID: 25996051 Free PMC article.
-
Role of IL-10 -819(t/c) promoter polymorphism in preeclampsia.Inflammation. 2014 Aug;37(4):1022-7. doi: 10.1007/s10753-014-9824-2. Inflammation. 2014. PMID: 24477695
-
Early pregnancy vitamin D status and risk of preeclampsia.J Clin Invest. 2016 Dec 1;126(12):4702-4715. doi: 10.1172/JCI89031. Epub 2016 Nov 14. J Clin Invest. 2016. PMID: 27841759 Free PMC article. Clinical Trial.
-
Hypoxia-Reoxygenation Impairs Autophagy-Lysosomal Machinery in Primary Human Trophoblasts Mimicking Placental Pathology of Early-Onset Preeclampsia.Int J Mol Sci. 2022 May 18;23(10):5644. doi: 10.3390/ijms23105644. Int J Mol Sci. 2022. PMID: 35628454 Free PMC article.
References
-
- Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–212. - PubMed
-
- Ilekis JV, Reddy UM, Roberts JM. Preeclampsia - A pressing problem: An executive summary of a National Institute of Child Health and Human Developmental Workshop. Reproductive Sciences. 2007;14:508–523. - PubMed
-
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep. 2006;55:1–101. - PubMed
-
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynecol. 1986;93:1049–1059. - PubMed
-
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

