Myasthenic syndrome caused by plectinopathy
- PMID: 21263134
- PMCID: PMC3034415
- DOI: 10.1212/WNL.0b013e31820882bd
Myasthenic syndrome caused by plectinopathy
Abstract
Background: Plectin crosslinks intermediate filaments to their targets in different tissues. Defects in plectin cause epidermolysis bullosa simplex (EBS), muscular dystrophy (MD), and sometimes pyloric atresia. Association of EBS with a myasthenic syndrome (MyS) was documented in a single patient in 1999.
Objectives: To analyze the clinical, structural, and genetic aspects of a second and fatal case of EBS associated with a MyS and search for the genetic basis of the disease in a previously reported patient with EBS-MD-MyS.
Methods: Clinical observations; histochemical, immunocytochemical, and electron microscopy studies of skeletal muscle and neuromuscular junction; and mutation analysis.
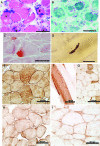
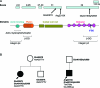
Results: An African American man had EBS since early infancy, and progressive muscle weakness, hyperCKemia, and myasthenic symptoms refractory to therapy since age 3 years. Eventually he became motionless and died at age 42 years. At age 15 years, he had a marked EMG decrement, and a reduced miniature endplate potential amplitude. The myopathy was associated with dislocated muscle fiber organelles, structurally abnormal nuclei, focal plasmalemmal defects, and focal calcium ingress into muscle fibers. The neuromuscular junctions showed destruction of the junctional folds, and remodeling. Mutation analysis demonstrated a known p.Arg2319X and a novel c.12043dupG mutation in PLEC1. The EBS-MD-MyS patient reported in 1999 also carried c.12043dupG and a novel p.Gln2057X mutation. The novel mutations were absent in 200 Caucasian and 100 African American subjects.
Conclusions: The MyS in plectinopathy is attributed to destruction of the junctional folds and the myopathy to defective anchoring of muscle fiber organelles and defects in sarcolemmal integrity.
Figures



References
-
- Elliott CE, Becker B, Oehler S, Castanon MJ, Hauptmann R, Wiche G. Plectin transcript diversity: identification and tissue distribution of variants with distinct first coding exons and rodless isoforms. Genomics 1997;42:115–125 - PubMed
-
- Fuchs P, Zorer M, Rezniczek GA, et al. Unusual 5′ transcript complexity of plectin isoforms: novel tissue-specific exons modulate actin binding activity. Hum Mol Genet 1999;8:2461–2472 - PubMed
-
- Banwell BL, Russel J, Fukudome T, Shen X-M, Stilling G, Engel AG. Myopathy, myasthenic syndrome, and epidermolysis bullosa simplex due to plectin deficiency. J Neuropathol Exp Neurol 1999;58:832–846 - PubMed
-
- McLean W, Pulkkinen L, Smith F, et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev 1996;10:1724–1735 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
