Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia
- PMID: 21263154
- PMCID: PMC3072880
- DOI: 10.1182/blood-2010-08-301267
Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia
Abstract
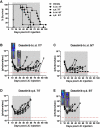
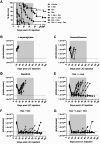
The introduction of cultured p185(BCR-ABL)-expressing (p185+) Arf (-/-) pre-B cells into healthy syngeneic mice induces aggressive acute lymphoblastic leukemia (ALL) that genetically and phenotypically mimics the human disease. We adapted this high-throughput Philadelphia chromosome-positive (Ph(+)) ALL animal model for in vivo luminescent imaging to investigate disease progression, targeted therapeutic response, and ALL relapse in living mice. Mice bearing high leukemic burdens (simulating human Ph(+) ALL at diagnosis) entered remission on maximally intensive, twice-daily dasatinib therapy, but invariably relapsed with disseminated and/or central nervous system disease. Although relapse was frequently accompanied by the eventual appearance of leukemic clones harboring BCR-ABL kinase domain (KD) mutations that confer drug resistance, their clonal emergence required prolonged dasatinib exposure. KD P-loop mutations predominated in mice receiving less intensive therapy, whereas high-dose treatment selected for T315I "gatekeeper" mutations resistant to all 3 Food and Drug Administration-approved BCR-ABL kinase inhibitors. The addition of dexamethasone and/or L-asparaginase to reduced-intensity dasatinib therapy improved long-term survival of the majority of mice that received all 3 drugs. Although non-tumor-cell-autonomous mechanisms can prevent full eradication of dasatinib-refractory ALL in this clinically relevant model, the emergence of resistance to BCR-ABL kinase inhibitors can be effectively circumvented by the addition of "conventional" chemotherapeutic agents with alternate antileukemic mechanisms of action.
Figures

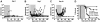


References
-
- Wong S, Witte ON. The BCR-ABL story: bench to bedside and back. Annu Rev Immunol. 2004;22:247–306. - PubMed
-
- Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

