FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo
- PMID: 21263155
- PMCID: PMC3069670
- DOI: 10.1182/blood-2010-01-266742
FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo
Abstract
We examined in vivo FLT3 inhibition in acute myeloid leukemia patients treated with chemotherapy followed by the FLT3 inhibitor lestaurtinib, comparing newly diagnosed acute myeloid leukemia patients with relapsed patients. Because we noted that in vivo FLT3 inhibition by lestaurtinib was less effective in the relapsed patients compared with the newly diagnosed patients, we investigated whether plasma FLT3 ligand (FL) levels could influence the efficacy of FLT3 inhibition in these patients. After intensive chemotherapy, FL levels rose to a mean of 488 pg/mL on day 15 of induction therapy for newly diagnosed patients, whereas they rose to a mean of 1148 pg/mL in the relapsed patients. FL levels rose even higher with successive courses of chemotherapy, to a mean of 3251 pg/mL after the fourth course. In vitro, exogenous FL at concentrations similar to those observed in patients mitigated FLT3 inhibition and cytotoxicity for each of 5 different FLT3 inhibitors (lestaurtinib, midostaurin, sorafenib, KW-2449, and AC220). The dramatic increase in FL level after chemotherapy represents a possible obstacle to inhibiting FLT3 in this clinical setting. These findings could have important implications regarding the design and outcome of trials of FLT3 inhibitors and furthermore suggest a rationale for targeting FL as a therapeutic strategy.
Figures


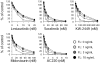


Comment in
-
Rethinking bioactivity of FLT3 inhibitors.Blood. 2011 Mar 24;117(12):3247-8. doi: 10.1182/blood-2011-02-334540. Blood. 2011. PMID: 21436074 No abstract available.
References
-
- Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. - PubMed
-
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. - PubMed
-
- Levis M, Small D. FLT3 tyrosine kinase inhibitors. Int J Hematol. 2005;82(2):100–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

