Transgenerational neuroendocrine disruption of reproduction
- PMID: 21263448
- PMCID: PMC3976559
- DOI: 10.1038/nrendo.2010.215
Transgenerational neuroendocrine disruption of reproduction
Abstract
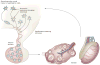
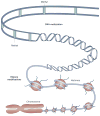
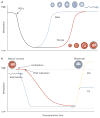
Exposure to endocrine disrupting chemicals (EDCs) is associated with dysfunctions of metabolism, energy balance, thyroid function and reproduction, and an increased risk of endocrine cancers. These multifactorial disorders can be 'programmed' through molecular epigenetic changes induced by exposure to EDCs early in life, the expression of which may not manifest until adulthood. In some cases, EDCs have detrimental effects on subsequent generations, which indicates that traits for disease predisposition may be passed to future generations by nongenomic inheritance. This Review discusses current understanding of the epigenetic mechanisms that underlie sexual differentiation of reproductive neuroendocrine systems in mammals and summarizes the literature on transgenerational epigenetic effects of representative EDCs: vinclozolin, diethylstilbesterol, bisphenol A and polychlorinated biphenyls. The article differentiates between context-dependent epigenetic transgenerational changes--namely, those that require environmental exposure, either via the EDC itself or through behavioral or physiological differences in parents--and germline-dependent epigenetic mechanisms. These processes, albeit discrete, are not mutually exclusive and can involve similar molecular mechanisms including DNA methylation and histone modifications and may predispose exposed individuals to transgenerational disruption of reproductive processes. New insights stress the crucial need to develop a clear understanding of how EDCs may program the epigenome of exposed individuals and their descendants.
© 2011 Macmillan Publishers Limited. All rights reserved
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Weybridge, UK, Report No. EUR 17549, Environment and Climate Research Programme, DG XXI. European Commission; Brussels: 1996.
-
- Kavlock RJ, Ankley GT. A perspective on the risk assessment process for endocrine-disruptive effects on wildlife and human health. Risk Anal. 1996;16:731–739. - PubMed
-
- Koistinen J, Stenman O, Haahti H, Suonperä M, Paasivirta J. Polychlorinated diphenyl ethers, dibenzo-p-dioxins, dibenzofurans and biphenyls in seals and sediment from the Gulf of Finland. Chemosphere. 1997;35:1249–1269. - PubMed
-
- Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

