Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States
- PMID: 21264063
- PMCID: PMC3025524
- DOI: 10.1128/mBio.00309-10
Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States
Abstract
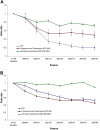
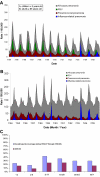
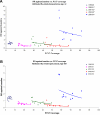
A seven-valent pneumococcal conjugate vaccine (PCV7) introduced in the United States in 2000 has been shown to reduce invasive pneumococcal disease (IPD) in both vaccinated children and adults through induction of herd immunity. We assessed the impact of infant immunization on pneumococcal pneumonia hospitalizations and mortality in all age groups using Health Care Utilization Project State Inpatient Databases (SID) for 1996 to 2006 from 10 states; SID contain 100% samples of ICD9-coded hospitalization data for the selected states. Compared to a 1996-1997 through 1998-1999 baseline, by the 2005-2006 season, both IPD and pneumococcal pneumonia hospitalizations and deaths had decreased substantially in all age groups, including a 47% (95% confidence interval [CI], 38 to 54%) reduction in nonbacteremic pneumococcal pneumonia (ICD9 code 481 with no codes indicating IPD) in infants <2 years old and a 54% reduction (CI, 53 to 56%) in adults ≥65 years of age. A model developed to calculate the total burden of pneumococcal pneumonia prevented by infant PCV7 vaccination in the United States from 2000 to 2006 estimated a reduction of 788,838 (CI, 695,406 to 875,476) hospitalizations for pneumococcal pneumonia. Ninety percent of the reduction in model-attributed pneumococcal pneumonia hospitalizations occurred through herd immunity among adults 18 years old and older; similar proportions were found in pneumococcal disease mortality prevented by the vaccine. In the first seasons after PCV introduction, when there were substantial state differences in coverage among <5-year-olds, states with greater coverage had significantly fewer influenza-associated pneumonia hospitalizations among children, suggesting that PCV7 use also reduces influenza-attributable pneumonia hospitalizations.
Copyright © 2011 Simonsen et al.
Figures
References
-
- Black R. E., Cousens S., Johnson H. L., Lawn J. E., Rudan I., Bassani D. G., Jha P., Campbell H., Walker C. F., Cibulskis R., Eisele T., Liu L., Mathers C. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987 - PubMed
-
- World Health Organization 2004. World health report. WHO, Geneva, Switzerland
-
- Klugman K. P., Madhi S. A., Huebner R. E., Kohberger R., Mbelle N., Pierce N. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341–1348 - PubMed
-
- Cutts F. T., Zaman S. M., Enwere G., Jaffar S., Levine O. S., Okoko J. B., Oluwalana C., Vaughan A., Obaro S. K., Leach A., McAdam K. P., Biney E., Saaka M., Onwuchekwa U., Yallop F., Pierce N. F., Greenwood B. M., Adegbola R. A. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365:1139–1146 - PubMed
-
- Black S., Shinefield H., Fireman B., Lewis E., Ray P., Hansen J. R., Elvin L., Ensor K. M., Hackell J., Siber G., Malinoski F., Madore D., Chang I., Kohberger R., Watson W., Austrian R., Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente vaccine study center group. Pediatr. Infect. Dis. J. 19:187–195 - PubMed

