Dynamics of macrophage trogocytosis of rituximab-coated B cells
- PMID: 21264210
- PMCID: PMC3022012
- DOI: 10.1371/journal.pone.0014498
Dynamics of macrophage trogocytosis of rituximab-coated B cells
Abstract
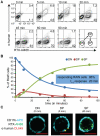
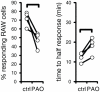
Macrophages can remove antigen from the surface of antibody-coated cells by a process termed trogocytosis. Using live cell microscopy and flow cytometry, we investigated the dynamics of trogocytosis by RAW264.7 macrophages of Ramos B cells opsonized with the anti-CD20 monoclonal antibody rituximab. Spontaneous and reversible formation of uropods was observed on Ramos cells, and these showed a strong enrichment in rituximab binding. RAW-Ramos conjugate interfaces were highly enriched in rituximab, and transfer of rituximab to the RAW cells in submicron-sized puncta occurred shortly after cell contact. Membrane from the target cells was concomitantly transferred along with rituximab to a variable extent. We established a flow cytometry-based approach to follow the kinetics of transfer and internalization of rituximab. Disruption of actin polymerization nearly eliminated transfer, while blocking phosphatidylinositol 3-kinase activity only resulted in a delay in its acquisition. Inhibition of Src family kinase activity both slowed acquisition and reduced the extent of trogocytosis. The effects of inhibiting these kinases are likely due to their role in efficient formation of cell-cell conjugates. Selective pre-treatment of Ramos cells with phenylarsine oxide blocked uropod formation, reduced enrichment of rituximab at cell-cell interfaces, and reduced the efficiency of trogocytic transfer of rituximab. Our findings highlight that dynamic changes in target cell shape and surface distribution of antigen may significantly influence the progression and extent of trogocytosis. Understanding the mechanistic determinants of macrophage trogocytosis will be important for optimal design of antibody therapies.
Conflict of interest statement
Figures

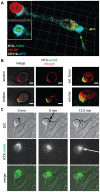
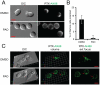
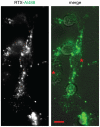
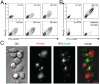


References
-
- Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–145. 10.1016/S0952-7915(01)00309-0. - PubMed
-
- Mero P, Zhang CY, Huang ZY, Kim MK, Schreiber AD, et al. Phosphorylation-independent Ubiquitylation and Endocytosis of Fc{gamma}RIIA. J Biol Chem. 2006;281:33242–33249. 10.1074/jbc.M605372200. - PubMed
-
- Tse SM, Furuya W, Gold E, Schreiber AD, Sandvig K, et al. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003;278:3331–3338. 10.1074/jbc.M207966200. - PubMed
-
- Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. 10.1038/ni0903-815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

