Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer's disease
- PMID: 21264269
- PMCID: PMC3020224
- DOI: 10.1371/journal.pone.0016032
Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer's disease
Abstract
Background: Ideally, disease modifying therapies for Alzheimer disease (AD) will be applied during the 'preclinical' stage (pathology present with cognition intact) before severe neuronal damage occurs, or upon recognizing very mild cognitive impairment. Developing and judiciously administering such therapies will require biomarker panels to identify early AD pathology, classify disease stage, monitor pathological progression, and predict cognitive decline. To discover such biomarkers, we measured AD-associated changes in the cerebrospinal fluid (CSF) proteome.
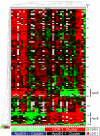
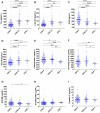
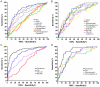
Methods and findings: CSF samples from individuals with mild AD (Clinical Dementia Rating [CDR] 1) (n = 24) and cognitively normal controls (CDR 0) (n = 24) were subjected to two-dimensional difference-in-gel electrophoresis. Within 119 differentially-abundant gel features, mass spectrometry (LC-MS/MS) identified 47 proteins. For validation, eleven proteins were re-evaluated by enzyme-linked immunosorbent assays (ELISA). Six of these assays (NrCAM, YKL-40, chromogranin A, carnosinase I, transthyretin, cystatin C) distinguished CDR 1 and CDR 0 groups and were subsequently applied (with tau, p-tau181 and Aβ42 ELISAs) to a larger independent cohort (n = 292) that included individuals with very mild dementia (CDR 0.5). Receiver-operating characteristic curve analyses using stepwise logistic regression yielded optimal biomarker combinations to distinguish CDR 0 from CDR>0 (tau, YKL-40, NrCAM) and CDR 1 from CDR<1 (tau, chromogranin A, carnosinase I) with areas under the curve of 0.90 (0.85-0.94 95% confidence interval [CI]) and 0.88 (0.81-0.94 CI), respectively.
Conclusions: Four novel CSF biomarkers for AD (NrCAM, YKL-40, chromogranin A, carnosinase I) can improve the diagnostic accuracy of Aβ42 and tau. Together, these six markers describe six clinicopathological stages from cognitive normalcy to mild dementia, including stages defined by increased risk of cognitive decline. Such a panel might improve clinical trial efficiency by guiding subject enrollment and monitoring disease progression. Further studies will be required to validate this panel and evaluate its potential for distinguishing AD from other dementing conditions.
Conflict of interest statement
Figures




References
-
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. - PubMed
-
- Morris J, Price J. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. - PubMed
-
- Price J, Ko A, Wade M, Tsou S, McKeel D, et al. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer's disease. Arch Neurol. 2001;58:1395–1402. - PubMed
-
- Markesbery W, Schmitt F, Kryscio R, Davis D, Smith C, et al. Neuropathologic substrate of Mild Cognitive Impairment. Arch Neurol. 2006;63:38–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG05136/AG/NIA NIH HHS/United States
- AG10124/AG/NIA NIH HHS/United States
- R01 AG023185/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- T32 NS007205/NS/NINDS NIH HHS/United States
- P41 RR00954/RR/NCRR NIH HHS/United States
- P41 RR000954/RR/NCRR NIH HHS/United States
- K23 AG020020/AG/NIA NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- P50 AG05681/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- AG23185/AG/NIA NIH HHS/United States
- P01 AG03991/AG/NIA NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- U01 AG16976/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- AG08017/AG/NIA NIH HHS/United States
- AG020020/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

