FimL regulates cAMP synthesis in Pseudomonas aeruginosa
- PMID: 21264306
- PMCID: PMC3019171
- DOI: 10.1371/journal.pone.0015867
FimL regulates cAMP synthesis in Pseudomonas aeruginosa
Abstract
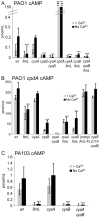
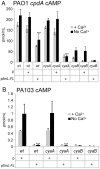
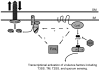
Pseudomonas aeruginosa, a ubiquitous bacteria found in diverse ecological niches, is an important cause of acute infections in immunocompromised individuals and chronic infections in patients with Cystic Fibrosis. One signaling molecule required for the coordinate regulation of virulence factors associated with acute infections is 3', 5'-cyclic adenosine monophosphate, (cAMP), which binds to and activates a catabolite repressor homolog, Vfr. Vfr controls the transcription of many virulence factors, including those associated with Type IV pili (TFP), the Type III secretion system (T3SS), the Type II secretion system, flagellar-mediated motility, and quorum sensing systems. We previously identified FimL, a protein with histidine phosphotransfer-like domains, as a regulator of Vfr-dependent processes, including TFP-dependent motility and T3SS function. In this study, we carried out genetic and physiologic studies to further define the mechanism of action of FimL. Through a genetic screen designed to identify suppressors of FimL, we found a putative cAMP-specific phosphodiesterase (CpdA), suggesting that FimL regulates cAMP levels. Inactivation of CpdA increases cAMP levels and restores TFP-dependent motility and T3SS function to fimL mutants, consistent with in vivo phosphodiesterase activity. By constructing combinations of double and triple mutants in the two adenylate cyclase genes (cyaA and cyaB), fimL, and cpdA, we show that ΔfimL mutants resemble ΔcyaB mutants in TM defects, decreased T3SS transcription, and decreased cAMP levels. Similar to some of the virulence factors that they regulate, we demonstrate that CyaB and FimL are polarly localized. These results reveal new complexities in the regulation of diverse virulence pathways associated with acute P. aeruginosa infections.
Conflict of interest statement
Figures




Similar articles
-
Pseudomonas aeruginosa fimL regulates multiple virulence functions by intersecting with Vfr-modulated pathways.Mol Microbiol. 2005 Mar;55(5):1357-78. doi: 10.1111/j.1365-2958.2005.04479.x. Mol Microbiol. 2005. PMID: 15720546 Free PMC article.
-
A scaffold protein connects type IV pili with the Chp chemosensory system to mediate activation of virulence signaling in Pseudomonas aeruginosa.Mol Microbiol. 2016 Aug;101(4):590-605. doi: 10.1111/mmi.13410. Epub 2016 May 27. Mol Microbiol. 2016. PMID: 27145134 Free PMC article.
-
The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity.Mol Microbiol. 2010 May;76(4):889-904. doi: 10.1111/j.1365-2958.2010.07135.x. Epub 2010 Mar 16. Mol Microbiol. 2010. PMID: 20345659 Free PMC article.
-
The multi-talented bacterial adenylate cyclases.Int J Med Microbiol. 2004 Apr;293(7-8):479-82. doi: 10.1078/1438-4221-00297. Int J Med Microbiol. 2004. PMID: 15149021 Review.
-
Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system.Mol Microbiol. 2006 Nov;62(3):631-40. doi: 10.1111/j.1365-2958.2006.05412.x. Epub 2006 Sep 21. Mol Microbiol. 2006. PMID: 16995895 Review.
Cited by
-
Phosphoryl Group Flow within the Pseudomonas aeruginosa Pil-Chp Chemosensory System: DIFFERENTIAL FUNCTION OF THE EIGHT PHOSPHOTRANSFERASE AND THREE RECEIVER DOMAINS.J Biol Chem. 2016 Aug 19;291(34):17677-91. doi: 10.1074/jbc.M116.737528. Epub 2016 Jun 27. J Biol Chem. 2016. PMID: 27354279 Free PMC article.
-
ChpC controls twitching motility-mediated expansion of Pseudomonas aeruginosa biofilms in response to serum albumin, mucin and oligopeptides.Microbiology (Reading). 2020 Jul;166(7):669-678. doi: 10.1099/mic.0.000911. Microbiology (Reading). 2020. PMID: 32478653 Free PMC article.
-
Evidence for the Type IV Pilus Retraction Motor PilT as a Component of the Surface Sensing System in Pseudomonas aeruginosa.J Bacteriol. 2023 Jul 25;205(7):e0017923. doi: 10.1128/jb.00179-23. Epub 2023 Jun 29. J Bacteriol. 2023. PMID: 37382531 Free PMC article.
-
Fitting Pieces into the Puzzle of Pseudomonas aeruginosa Type III Secretion System Gene Expression.J Bacteriol. 2019 Jun 10;201(13):e00209-19. doi: 10.1128/JB.00209-19. Print 2019 Jul 1. J Bacteriol. 2019. PMID: 31010903 Free PMC article. Review.
-
The Cyclic AMP-Vfr Signaling Pathway in Pseudomonas aeruginosa Is Inhibited by Cyclic Di-GMP.J Bacteriol. 2015 Jul;197(13):2190-200. doi: 10.1128/JB.00193-15. Epub 2015 Apr 20. J Bacteriol. 2015. PMID: 25897033 Free PMC article.
References
-
- Mandell GL, Bennett JE, Dolin R. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed. 1 online resource (2 v. (cl, 4028, xcvii p.))
-
- Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009;12:61–66. - PubMed
-
- Mattick JS. Type iv pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. - PubMed

