Bystanding F+ oxidants enable selective reductive elimination from high-valent metal centers in catalysis
- PMID: 21264991
- PMCID: PMC3094160
- DOI: 10.1002/anie.201005142
Bystanding F+ oxidants enable selective reductive elimination from high-valent metal centers in catalysis
Abstract
Reductive elimination from partially or completely oxidized metal centers is a vital step in a myriad of carbon-carbon and carbon-heteroatom bond-forming reactions. One strategy for promoting otherwise challenging reductive elimination reactions is to oxidize the metal center using a two-electron oxidant (that is, from M((n)) to M((n+2))). However, many of the commonly used oxidants for this type of transformation contain oxygen, nitrogen, or halogen moieties that are subsequently capable of participating in reductive elimination, thus leading to a mixture of products. In this Minireview, we examine the use of bystanding F(+) oxidants for addressing this widespread problem in organometallic chemistry and describe recent applications in Pd(II) /Pd(IV) and Au(I) /Au(III) catalysis. We then briefly discuss a rare example in which one-electron oxidants have been shown to promote selective reductive elimination in palladium(II)-catalyzed C-H functionalization, which we view as a promising future direction in the field.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


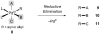

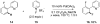
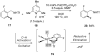
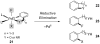

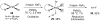


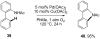
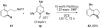
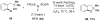


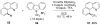

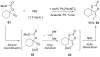
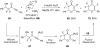


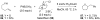

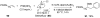

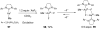
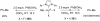

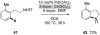


References
-
- Gillie A, Stille JK. J Am Chem Soc. 1980;102:4933–4941.
-
-
For reviews of C–Y reductive elimination from Pd(II) centers, see: Hartwig JF. Acc Chem Res. 1998;31:852–860.Hartwig JF. Nature. 2008;455:314–322.
-
-
-
For reviews discussing ligand design in Pd(0) chemistry, see: Herrmann WA. Angew Chem. 2002;114:1342–1363.Angew Chem Int Ed. 2002;41:1290–1309.Littke AF, Fu GC. Angew Chem. 2002;114:4350–4386.Angew Chem Int Ed. 2002;41:4176–4211.Martin R, Buchwald SL. Acc Chem Res. 2008;41:1461–1473.Hartwig JF. Acc Chem Res. 2008;41:1534–1544.
-
-
-
For reviews of Pd-catalyzed C–H functionalization reactions, see: Jia C, Kitamura T, Fujiwara Y. Acc Chem Res. 2001;34:633–639.Satoh T, Miura M. Chem Lett. 2007;36:200–205.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173–1193.Campeau LC, Stuart DR, Fagnou K. Aldrichim Acta. 2007;40:35–41.Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174–238.Li BJ, Yang SD, Shi ZJ. Synlett. 2008:949–957.Kakiuchi F, Kochi T. Synthesis. 2008:3013–3039.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074–1086.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem. 2009;121:5196–5217.Angew Chem Int Ed. 2009;48:5094–5115.Ackermann L, Vicente R, Kapdi AR. Angew Chem. 2009;121:9976–10011.Angew Chem Int Ed. 2009;48:9792–9826.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147–1169.
-
-
-
Hegedus LS. Transition Metals in the Synthesis of Complex Organic Molecules. 2nd. University Science Books; Sausalito (CA, USA): 1999. pp. 22–23. For examples of CO-promoted reductive elimination in transition metal chemistry, see: Kondo T, Kodoi K, Nishinaga E, Okada T, Morisaki Y, Watanabe Y, Mitsudo T. J Am Chem Soc. 1998;120:5587–5588.Carbayo A, Cuevas JV, García-Herbosa G. J Organomet Chem. 2002;658:15–20.West NM, Reinartz S, White PS, Templeton JL. J Am Chem Soc. 2006;128:2059–2066.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

