Microarray-based identification of tomato microRNAs and time course analysis of their response to Cucumber mosaic virus infection
- PMID: 21265043
- PMCID: PMC3030956
- DOI: 10.1631/jzus.B1000278
Microarray-based identification of tomato microRNAs and time course analysis of their response to Cucumber mosaic virus infection
Abstract
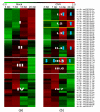
A large number of plant microRNAs (miRNAs) are now documented in the miRBase, among which only 30 are for Solanum lycopersicum (tomato). Clearly, there is a far-reaching need to identify and profile the expression of miRNAs in this important crop under various physiological and pathological conditions. In this study, we used an in situ synthesized custom microarray of plant miRNAs to examine the expression and temporal presence of miRNAs in the leaves of tomato plants infected with Cucumber mosaic virus (CMV). Following computational sequence homology search and hairpin structure prediction, we identified three novel tomato miRNA precursor genes. Our results also show that, in accordance with the phenotype of the developing leaves, the tomato miRNAs are differentially expressed at different stages of plant development and that CMV infection can induce or suppress the expression of miRNAs as well as up-regulate some star miRNAs (miRNA*s) which are normally present at much lower levels. The results indicate that developmental anomalies elicited by virus infection may be caused by more complex biological processes.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

