Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum
- PMID: 21266052
- PMCID: PMC3041658
- DOI: 10.1186/1472-6750-11-9
Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum
Abstract
Background: Laccases are multi-copper oxidases that catalyze the one electron oxidation of a broad range of compounds. Laccase substrates include substituted phenols, arylamines and aromatic thiols. Such compounds are activated by the enzyme to the corresponding radicals. Owing to their broad substrate range laccases are considered to be versatile biocatalysts which are capable of oxidizing natural and non-natural industrial compounds, with water as sole by-product.
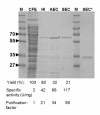
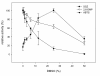
Results: A novel CotA-type laccase from Bacillus pumilus was cloned, expressed and purified and its biochemical characteristics are presented here. The molecular weight of the purified laccase was estimated to be 58 kDa and the enzyme was found to be associated with four copper atoms. Its catalytic activity towards 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 2,6-dimethoxyphenol (2,6-DMP) and syringaldazine (SGZ) was investigated. The kinetic parameters KM and kcat for ABTS were 80 ± 4 μM and 291 ± 2.7 s(-1), for 2,6-DMP 680 ± 27 μM and 11 ± 0.1 s(-1) and for SGZ only kcat could be estimated to be 66 ± 1.5 s(-1). The pH optimum for ABTS was 4, for 2,6-DMP 7 and for SGZ 6.5 and temperature optima for ABTS and 2,6-DMP were found to be around 70°C. The screening of 37 natural and non-natural compounds as substrates for B. pumilus laccase revealed 18 suitable compounds. Three of them served as redox mediators in the laccase-catalyzed decolorization of the dye indigocarmine (IC), thus assessing the new enzyme's biotechnological potential.
Conclusions: The fully copper loaded, thermostable CotA laccase from Bacillus pumilus is a versatile laccase with potential applications as an industrial biocatalyst.
Figures

Similar articles
-
Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids.Appl Microbiol Biotechnol. 2008 May;79(2):217-24. doi: 10.1007/s00253-008-1417-2. Appl Microbiol Biotechnol. 2008. PMID: 18330561
-
Enhanced expression of a recombinant bacterial laccase at low temperature and microaerobic conditions: purification and biochemical characterization.J Ind Microbiol Biotechnol. 2010 Aug;37(8):863-9. doi: 10.1007/s10295-010-0734-5. Epub 2010 May 15. J Ind Microbiol Biotechnol. 2010. PMID: 20473548
-
Biochemical and Structural Characterization of a Novel Psychrophilic Laccase (Multicopper Oxidase) Discovered from Oenococcus oeni 229 (ENOLAB 4002).Int J Mol Sci. 2024 Aug 5;25(15):8521. doi: 10.3390/ijms25158521. Int J Mol Sci. 2024. PMID: 39126090 Free PMC article.
-
Laccase: a multi-purpose biocatalyst at the forefront of biotechnology.Microb Biotechnol. 2017 Nov;10(6):1457-1467. doi: 10.1111/1751-7915.12422. Epub 2016 Oct 3. Microb Biotechnol. 2017. PMID: 27696775 Free PMC article. Review.
-
Laccases: Versatile Biocatalysts for the Synthesis of Heterocyclic Cores.Molecules. 2021 Jun 18;26(12):3719. doi: 10.3390/molecules26123719. Molecules. 2021. PMID: 34207073 Free PMC article. Review.
Cited by
-
Bilirubin oxidase from Bacillus pumilus: a promising enzyme for the elaboration of efficient cathodes in biofuel cells.Biosens Bioelectron. 2012 May 15;35(1):140-146. doi: 10.1016/j.bios.2012.02.033. Epub 2012 Feb 25. Biosens Bioelectron. 2012. PMID: 22410485 Free PMC article.
-
Trp-His covalent adduct in bilirubin oxidase is crucial for effective bilirubin binding but has a minor role in electron transfer.Sci Rep. 2019 Sep 23;9(1):13700. doi: 10.1038/s41598-019-50105-3. Sci Rep. 2019. PMID: 31548583 Free PMC article.
-
Degradation of Aflatoxin B1 and Zearalenone by Bacterial and Fungal Laccases in Presence of Structurally Defined Chemicals and Complex Natural Mediators.Toxins (Basel). 2019 Oct 22;11(10):609. doi: 10.3390/toxins11100609. Toxins (Basel). 2019. PMID: 31652557 Free PMC article.
-
Expression, secretion and functional characterization of three laccases in E. coli.Synth Syst Biotechnol. 2021 Dec 7;7(1):474-480. doi: 10.1016/j.synbio.2021.12.002. eCollection 2022 Mar. Synth Syst Biotechnol. 2021. PMID: 34938906 Free PMC article.
-
Aptitude of Oxidative Enzymes for Treatment of Wastewater Pollutants: A Laccase Perspective.Molecules. 2019 May 30;24(11):2064. doi: 10.3390/molecules24112064. Molecules. 2019. PMID: 31151229 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

