Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice
- PMID: 21266194
- PMCID: PMC3098320
- DOI: 10.1016/j.bbi.2011.01.008
Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice
Abstract
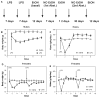
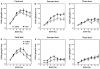
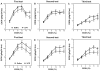
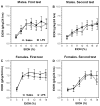
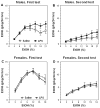
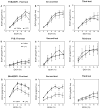
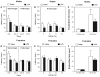
Previous studies showed that mice with genetic predisposition for high alcohol consumption as well as human alcoholics show changes in brain expression of genes related to immune signaling. In addition, mutant mice lacking genes related to immune function show decreased alcohol consumption (Blednov et al., 2011), suggesting that immune signaling promotes alcohol consumption. To test the possibility that activation of immune signaling will increase alcohol consumption, we treated mice with lipopolysaccaride (LPS; 1mg/kg, i.p.) and tested alcohol consumption in the continuous two-bottle choice test. To take advantage of the long-lasting activation of brain immune signaling by LPS, we measured drinking beginning one week or one month after LPS treatment and continued the studies for several months. LPS produced persistent increases in alcohol consumption in C57BL/6J (B6) inbred mice, FVBxB6F1 and B6xNZBF1 hybrid mice, but not in FVB inbred mice. To determine if this effect of LPS is mediated through binding to TLR4, we tested mice lacking CD14, a key component of TLR4 signaling. These null mutants showed no increase of alcohol intake after treatment with LPS. LPS treatment decreased ethanol-conditioned taste aversion but did not alter ethanol-conditioned place preference (B6xNZBF1 mice). Electrophysiological studies of dopamine neurons in the ventral tegmental area showed that pretreatment of mice with LPS decreased the neuronal firing rate. These results suggest that activation of immune signaling promotes alcohol consumption and alters certain aspects of alcohol reward/aversion.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Aubert A, Dantzer R. The taste of sickness: lipopolysaccharide-induced finickiness in rats. Physiol Behav. 2005;84:437–444. - PubMed
-
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. - PubMed
-
- Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

