Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia
- PMID: 21266262
- PMCID: PMC3381791
- DOI: 10.1016/j.semnephrol.2010.10.003
Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia
Abstract
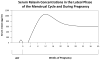
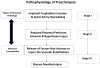
Relaxin is an approximately 6-kilodalton peptide hormone secreted by the corpus luteum, and circulates in the maternal blood during pregnancy. Relaxin administration to awake, chronically instrumented, nonpregnant rats mimics the vasodilatory phenomena of pregnancy. Furthermore, immunoneutralization of relaxin or its elimination from the circulation during midterm pregnancy in awake rats prevents maternal systemic and renal vasodilation, and the increase in global arterial compliance. Human investigation, albeit limited through 2010, also reveals vasodilatory effects of relaxin in the nonpregnant condition and observations consistent with a role for relaxin in gestational renal hyperfiltration. Evidence suggests that the vasodilatory responses of relaxin are mediated by its major receptor, the relaxin/insulin-like family peptide 1 receptor, RFXP1. The molecular mechanisms of relaxin vasodilation depend on the duration of hormone exposure (ie, there are rapid and sustained vasodilatory responses). Newly emerging data support the role of Gα(i/o) protein coupling to phosphatidylinositol-3 kinase/Akt (protein kinase B)-dependent phosphorylation and activation of endothelial nitric oxide synthase in the rapid vasodilatory responses of relaxin. Sustained vasodilatory responses critically depend on vascular endothelial and placental growth factors, and increases in arterial gelatinase(s) activity. Gelatinases hydrolyze big endothelin (ET) at a gly-leu bond to form ET(1-32), which activates the endothelial ET(B)/nitric oxide vasodilatory pathway. Although the relevance of relaxin biology to preeclampsia is largely speculative at this time, there are potential tantalizing links that are discussed in the context of our current understanding of the etiology and pathophysiology of the disease.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Sherwood OD. Relaxin. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 861–1008.
-
- Kohsaka T, Min G, Lukas G, Trupin S, Campbell ET, Sherwood OD. Identification of specific relaxin-binding cells in the human female. Biol Reprod. 1998;59:991–9. - PubMed
-
- Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, et al. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J. 2006;20:2352–62. - PubMed
-
- Hisaw FL. Experimental relaxation of the public ligament of the guinea pig. Proc Exp Biol Med. 1926;23:661–3.
-
- Ziel HK, Swain CT, Frederick L. Hisaw and the discovery of relaxin. Endocrinologist. 2000;10:215–8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

