Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas
- PMID: 21266405
- PMCID: PMC3026412
- DOI: 10.1242/dev.056499
Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas
Abstract
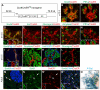
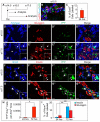
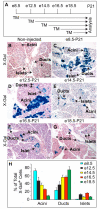
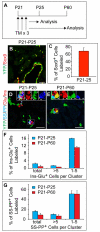
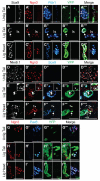
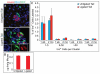
One major unresolved question in the field of pancreas biology is whether ductal cells have the ability to generate insulin-producing β-cells. Conclusive examination of this question has been limited by the lack of appropriate tools to efficiently and specifically label ductal cells in vivo. We generated Sox9CreER(T2) mice, which, during adulthood, allow for labeling of an average of 70% of pancreatic ductal cells, including terminal duct/centroacinar cells. Fate-mapping studies of the Sox9(+) domain revealed endocrine and acinar cell neogenesis from Sox9(+) cells throughout embryogenesis. Very small numbers of non-β endocrine cells continue to arise from Sox9(+) cells in early postnatal life, but no endocrine or acinar cell neogenesis from Sox9(+) cells occurs during adulthood. In the adult pancreas, pancreatic injury by partial duct ligation (PDL) has been suggested to induce β-cell regeneration from a transient Ngn3(+) endocrine progenitor cell population. Here, we identify ductal cells as a cell of origin for PDL-induced Ngn3(+) cells, but fail to observe β-cell neogenesis from duct-derived cells. Therefore, although PDL leads to activation of Ngn3 expression in ducts, PDL does not induce appropriate cues to allow for completion of the entire β-cell neogenesis program. In conclusion, although endocrine cells arise from the Sox9(+) ductal domain throughout embryogenesis and the early postnatal period, Sox9(+) ductal cells of the adult pancreas no longer give rise to endocrine cells under both normal conditions and in response to PDL.
Figures



References
-
- Cheung M., Briscoe J. (2003). Neural crest development is regulated by the transcription factor Sox9. Development 130, 5681-5693 - PubMed
-
- Chung C. H., Hao E., Piran R., Keinan E., Levine F. (2010). Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells 28, 1630-1638 - PubMed
-
- Dor Y., Brown J., Martinez O. I., Melton D. A. (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41-46 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

