Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds
- PMID: 21266457
- PMCID: PMC3115578
- DOI: 10.1093/hmg/ddr029
Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds
Abstract
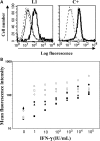
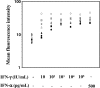
We report a series of 14 patients from 11 kindreds with recessive partial (RP)-interferon (IFN)-γR1 deficiency. The I87T mutation was found in nine homozygous patients from Chile, Portugal and Poland, and the V63G mutation was found in five homozygous patients from the Canary Islands. Founder effects accounted for the recurrence of both mutations. The most recent common ancestors of the patients with the I87T and V63G mutations probably lived 1600 (875-2950) and 500 (200-1275) years ago, respectively. The two alleles confer phenotypes that are similar but differ in terms of IFN-γR1 levels and residual response to IFN-γ. The patients suffered from bacillus Calmette-Guérin-osis (n= 6), environmental mycobacteriosis (n= 6) or tuberculosis (n= 1). One patient did not suffer from mycobacterial infections but had disseminated salmonellosis, which was also present in two other patients. Age at onset of the first environmental mycobacterial disease differed widely between patients, with a mean value of 11.25 ± 9.13 years. Thirteen patients survived until the age of 14.82 ± 11.2 years, and one patient died at the age of 7 years, 9 days after the diagnosis of long-term Mycobacterium avium infection and the initiation of antimycobacterial treatment. Up to 10 patients are currently free of infection with no prophylaxis. The clinical heterogeneity of the 14 patients was not clearly related to either IFNGR1 genotype or the resulting cellular phenotype. RP-IFN-γR1 deficiency is, thus, more common than initially thought and should be considered in both children and adults with mild or severe mycobacterial diseases.
Figures






References
-
- Casanova J.L., Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317:617–619. doi:10.1126/science.1142963. - DOI - PubMed
-
- Casanova J.L., Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 2002;20:581–620. doi:10.1146/annurev.immunol.20.081501.125851. - DOI - PubMed
-
- Rosenzweig S.D., Holland S.M. Defects in the interferon-gamma and interleukin-12 pathways. Immunol. Rev. 2005;203:38–47. doi:10.1111/j.0105-2896.2005.00227.x. - DOI - PubMed
-
- Filipe-Santos O., Bustamante J., Chapgier A., Vogt G., de Beaucoudrey L., Feinberg J., Jouanguy E., Boisson-Dupuis S., Fieschi C., Picard C., et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol. 2006;18:347–361. doi:10.1016/j.smim.2006.07.010. - DOI - PubMed
-
- Alcais A., Fieschi C., Abel L., Casanova J.L. Tuberculosis in children and adults: two distinct genetic diseases. J. Exp. Med. 2005;202:1617–1621. doi:10.1084/jem.20071987. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

