Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism
- PMID: 21266573
- PMCID: PMC3058958
- DOI: 10.1074/jbc.M110.180638
Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism
Abstract
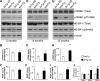
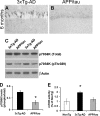
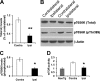
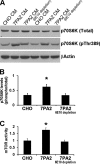
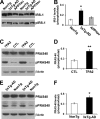
Reducing the mammalian target of rapamycin (mTOR) activity increases lifespan and health span in a variety of organisms. Alterations in protein homeostasis and mTOR activity and signaling have been reported in several neurodegenerative disorders, including Alzheimer disease (AD); however, the causes of such deregulations remain elusive. Here, we show that mTOR activity and signaling are increased in cell lines stably transfected with mutant amyloid precursor protein (APP) and in brains of 3xTg-AD mice, an animal model of AD. In addition, we show that in the 3xTg-AD mice, mTOR activity can be reduced to wild type levels by genetically preventing Aβ accumulation. Similarly, intrahippocampal injections of an anti-Aβ antibody reduced Aβ levels and normalized mTOR activity, indicating that high Aβ levels are necessary for mTOR hyperactivity in 3xTg-AD mice. We also show that the intrahippocampal injection of naturally secreted Aβ is sufficient to increase mTOR signaling in the brains of wild type mice. The mechanism behind the Aβ-induced mTOR hyperactivity is mediated by the proline-rich Akt substrate 40 (PRAS40) as we show that the activation of PRAS40 plays a key role in the Aβ-induced mTOR hyperactivity. Taken together, our data show that Aβ accumulation, which has been suggested to be the culprit of AD pathogenesis, causes mTOR hyperactivity by regulating PRAS40 phosphorylation. These data further indicate that the mTOR pathway is one of the pathways by which Aβ exerts its toxicity and further support the idea that reducing mTOR signaling in AD may be a valid therapeutic approach.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

