A novel putrescine importer required for type 1 pili-driven surface motility induced by extracellular putrescine in Escherichia coli K-12
- PMID: 21266585
- PMCID: PMC3060471
- DOI: 10.1074/jbc.M110.176032
A novel putrescine importer required for type 1 pili-driven surface motility induced by extracellular putrescine in Escherichia coli K-12
Abstract
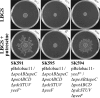
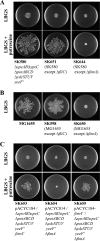
Recently, many studies have reported that polyamines play a role in bacterial cell-to-cell signaling processes. The present study describes a novel putrescine importer required for induction of type 1 pili-driven surface motility. The surface motility of the Escherichia coli ΔspeAB ΔspeC ΔpotABCD strain, which cannot produce putrescine and cannot import spermidine from the medium, was induced by extracellular putrescine. Introduction of the gene deletions for known polyamine importers (ΔpotE, ΔpotFGHI, and ΔpuuP) or a putative polyamine importer (ΔydcSTUV) into the ΔspeAB ΔspeC ΔpotABCD strain did not affect putrescine-induced surface motility. The deletion of yeeF, an annotated putative putrescine importer, in the ΔspeAB ΔspeC ΔpotABCD ΔydcSTUV strain abolished surface motility in putrescine-supplemented medium. Complementation of yeeF by a plasmid vector restored surface motility. The surface motility observed in the present study was abolished by the deletion of fimA, suggesting that the surface motility is type 1 pili-driven. A transport assay using the yeeF(+) or ΔyeeF strains revealed that YeeF is a novel putrescine importer. The K(m) of YeeF (155 μM) is 40 to 300 times higher than that of other importers reported previously. On the other hand, the V(max) of YeeF (9.3 nmol/min/mg) is comparable to that of PotABCD, PotFGHI, and PuuP. The low affinity of YeeF for putrescine may allow E. coli to sense the cell density depending on the concentration of extracellular putrescine.
Figures




References
-
- Gerner E. W., Meyskens F. L., Jr. (2004) Nat. Rev. Cancer 4, 781–792 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

