Defective Tbx2-dependent patterning of the atrioventricular canal myocardium causes accessory pathway formation in mice
- PMID: 21266775
- PMCID: PMC3026729
- DOI: 10.1172/JCI44350
Defective Tbx2-dependent patterning of the atrioventricular canal myocardium causes accessory pathway formation in mice
Abstract
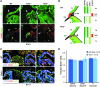
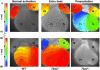
Ventricular preexcitation, a feature of Wolff-Parkinson-White syndrome, is caused by accessory myocardial pathways that bypass the annulus fibrosus. This condition increases the risk of atrioventricular tachycardia and, in the presence of atrial fibrillation, sudden death. The developmental mechanisms underlying accessory pathway formation are poorly understood but are thought to primarily involve malformation of the annulus fibrosus. Before birth, slowly conducting atrioventricular myocardium causes a functional atrioventricular activation delay in the absence of the annulus fibrosus. This myocardium remains present after birth, suggesting that the disturbed development of the atrioventricular canal myocardium may mediate the formation of rapidly conducting accessory pathways. Here we show that myocardium-specific inactivation of T-box 2 (Tbx2), a transcription factor essential for atrioventricular canal patterning, leads to the formation of fast-conducting accessory pathways, malformation of the annulus fibrosus, and ventricular preexcitation in mice. The accessory pathways ectopically express proteins required for fast conduction (connexin-40 [Cx40], Cx43, and sodium channel, voltage-gated, type V, α [Scn5a]). Additional inactivation of Cx30.2, a subunit for gap junctions with low conductance expressed in the atrioventricular canal and unaffected by the loss of Tbx2, did not affect the functionality of the accessory pathways. Our results suggest that malformation of the annulus fibrosus and preexcitation arise from the disturbed development of the atrioventricular myocardium.
Figures




Comment in
-
Navigational error in the heart leads to premature ventricular excitation.J Clin Invest. 2011 Feb;121(2):513-6. doi: 10.1172/JCI46038. Epub 2011 Jan 25. J Clin Invest. 2011. PMID: 21266771 Free PMC article.
References
-
- Munger TM, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993;87(3):866–873. - PubMed
-
- Packard JM, Graettinger JS, Graybiel A. Analysis of the electrocardiograms obtained from 1000 young healthy aviators; ten year follow-up. Circulation. 1954;10(3):384–400. - PubMed
-
- Centurion OA, Shimizu A, Isomoto S, Konoe A. Mechanisms for the genesis of paroxysmal atrial fibrillation in the Wolff Parkinson-White syndrome: intrinsic atrial muscle vulnerability vs. electrophysiological properties of the accessory pathway. Europace. 2008;10(3):294–302. doi: 10.1093/europace/eun031. - DOI - PubMed
-
- Basso C, Corrado D, Rossi L, Thiene G. Ventricular preexcitation in children and young adults: atrial myocarditis as a possible trigger of sudden death. Circulation. 2001;103(2):269–275. - PubMed
-
- Wood FC, Wolferth CC, Geckeler GD. Histologic demonstration of accessory muscular connections between auricle and ventricle in a case of short P-R interval and prolonged QRS complex. Am Heart J. 1943;25(4):454–462. doi: 10.1016/S0002-8703(43)90484-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

