Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways
- PMID: 21266778
- PMCID: PMC3026731
- DOI: 10.1172/JCI44470
Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways
Abstract
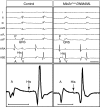
Ventricular preexcitation, which characterizes Wolff-Parkinson-White syndrome, is caused by the presence of accessory pathways that can rapidly conduct electrical impulses from atria to ventricles, without the intrinsic delay characteristic of the atrioventricular (AV) node. Preexcitation is associated with an increased risk of tachyarrhythmia, palpitations, syncope, and sudden death. Although the pathology and electrophysiology of preexcitation syndromes are well characterized, the developmental mechanisms are poorly understood, and few animal models that faithfully recapitulate the human disorder have been described. Here we show that activation of Notch signaling in the developing myocardium of mice can produce fully penetrant accessory pathways and ventricular preexcitation. Conversely, inhibition of Notch signaling in the developing myocardium resulted in a hypoplastic AV node, with specific loss of slow-conducting cells expressing connexin-30.2 (Cx30.2) and a resulting loss of physiologic AV conduction delay. Taken together, our results suggest that Notch regulates the functional maturation of AV canal embryonic myocardium during the development of the specialized conduction system. Our results also show that ventricular preexcitation can arise from inappropriate patterning of the AV canal-derived myocardium.
Figures





Comment in
-
Navigational error in the heart leads to premature ventricular excitation.J Clin Invest. 2011 Feb;121(2):513-6. doi: 10.1172/JCI46038. Epub 2011 Jan 25. J Clin Invest. 2011. PMID: 21266771 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

