Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial
- PMID: 21267072
- PMCID: PMC3022577
- DOI: 10.1371/journal.pone.0014501
Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial
Abstract
Background: New antimalarials are needed for P. vivax and P. falciparum malaria. This study compared the efficacy and safety of pyronaridine-artesunate with that of chloroquine for the treatment of uncomplicated P. vivax malaria.
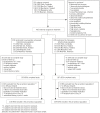
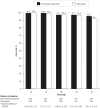
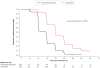
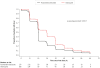
Methods and findings: This phase III randomized, double-blind, non-inferiority trial included five centers across Cambodia, Thailand, India, and Indonesia. In a double-dummy design, patients (aged >3-≤ 60 years) with microscopically confirmed P. vivax mono-infection were randomized (1:1) to receive pyronaridine-artesunate (target dose 7.2:2.4 mg/kg to 13.8:4.6 mg/kg) or chloroquine (standard dose) once daily for three days. Each treatment group included 228 randomized patients. Outcomes for the primary endpoint, Day-14 cure rate in the per-protocol population, were 99.5%, (217/218; 95%CI 97.5, 100) with pyronaridine-artesunate and 100% (209/209; 95%CI 98.3, 100) with chloroquine. Pyronaridine was non-inferior to chloroquine: treatment difference -0.5% (95%CI -2.6, 1.4), i.e., the lower limit of the 2-sided 95%CI for the treatment difference was greater than -10%. Pyronaridine-artesunate cure rates were non-inferior to chloroquine for Days 21, 28, 35 and 42. Parasite clearance time was shorter with pyronaridine-artesunate (median 23.0 h) versus chloroquine (32.0 h; p<0.0001), as was fever clearance time (median 15.9 h and 23.8 h, respectively; p = 0.0017). Kaplan-Meier estimates of post-baseline P. falciparum infection incidence until Day 42 were 2.5% with pyronaridine-artesunate, 6.1% with chloroquine (p = 0.048, log-rank test). Post-baseline P. vivax or P. falciparum infection incidence until Day 42 was 6.8% and 12.4%, respectively (p = 0.022, log rank test). There were no deaths. Adverse events occurred in 92/228 (40.4%) patients with pyronaridine-artesunate and 72/228 (31.6%) with chloroquine. Mild and transient increases in hepatic enzymes were observed for pyronaridine-artesunate.
Conclusion: Pyronaridine-artesunate efficacy in acute uncomplicated P. vivax malaria was at least that of chloroquine. As pyronaridine-artesunate is also efficacious against P. falciparum malaria, this combination has potential utility as a global antimalarial drug.
Trial registration: Clinicaltrials.gov NCT00440999.
Conflict of interest statement
Figures

References
-
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. - PubMed
-
- Parakh A, Agarwal N, Aggarwal A, Aneja A. Plasmodium vivax malaria in children: uncommon manifestations. Ann Trop Paediatr. 2009;29:253–256. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

