Japanese encephalitis Virus wild strain infection suppresses dendritic cells maturation and function, and causes the expansion of regulatory T cells
- PMID: 21269456
- PMCID: PMC3038949
- DOI: 10.1186/1743-422X-8-39
Japanese encephalitis Virus wild strain infection suppresses dendritic cells maturation and function, and causes the expansion of regulatory T cells
Abstract
Background: Japanese encephalitis (JE) caused by Japanese encephalitis virus (JEV) accounts for acute illness and death. However, few studies have been conducted to unveil the potential pathogenesis mechanism of JEV. Dendritic cells (DCs) are the most prominent antigen-presenting cells (APCs) which induce dual humoral and cellular responses. Thus, the investigation of the interaction between JEV and DCs may be helpful for resolving the mechanism of viral escape from immune surveillance and JE pathogenesis.
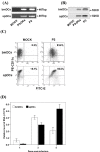
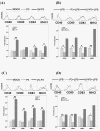
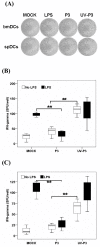
Results: We examined the alterations of phenotype and function of DCs including bone marrow-derived DCs (bmDCs) in vitro and spleen-derived DCs (spDCs) in vivo due to JEV P3 wild strain infection. Our results showed that JEV P3 infected DCs in vitro and in vivo. The viral infection inhibited the expression of cell maturation surface markers (CD40, CD80 and CD83) and MHCⅠ, and impaired the ability of P3-infected DCs for activating allogeneic naïve T cells. In addition, P3 infection suppressed the expression of interferon (IFN)-α and tumor necrosis factor (TNF)-α but enhanced the production of chemokine (C-C motif) ligand 2 (CCL2) and interleukin (IL)-10 of DCs. The infected DCs expanded the population of CD4+ Foxp3+ regulatory T cell (Treg).
Conclusion: JEV P3 infection of DCs impaired cell maturation and T cell activation, modulated cytokine productions and expanded regulatory T cells, suggesting a possible mechanism of JE development.
Figures



References
-
- Hanna JN, Ritchie SA, Phillips DA, Shield J, Bailey MC, Mackenzie JS, Poidinger M, McCall BJ, Mills PJ. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust. 1996;165:256–260. - PubMed
-
- Ravi V, Parida S, Desai A, Chandramuki A, Gourie-Devi M, Grau GE. Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J Med Virol. 1997;51:132–136. doi: 10.1002/(SICI)1096-9071(199702)51:2<132::AID-JMV8>3.0.CO;2-8. - DOI - PubMed
-
- Singh A, Kulshreshtha R, Mathur A. Secretion of the chemokine interleukin-8 during Japanese encephalitis virus infection. J Med Microbiol. 2000;49:607–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

