Vasculoprotective effects of rosiglitazone through modulating renin-angiotensin system in vivo and vitro
- PMID: 21269478
- PMCID: PMC3039565
- DOI: 10.1186/1475-2840-10-10
Vasculoprotective effects of rosiglitazone through modulating renin-angiotensin system in vivo and vitro
Abstract
Background: The peroxisome proliferator-activated receptor-γ (PPARγ) agonist rosiglitazone has been suggested to exert cardiovascular protection through the improvement of lipid metabolism, anti-inflammation, anti-proliferation etc. However, whether renin-angiotensin system (RAS) is involved in the vascular protective effects of PPARγ agonists is not fully understood. The present study aimed to investigate the effects of the renin-angiotensin system in vascular protection mediated by PPARγ agonists.
Objective: To investigate the actions of the renin-angiotensin system in vascular protection mediated by activation of PPARγ in vivo and in vitro.
Methods: Rats were fed a regular diet (n = 8), a cholesterol-rich diet plus methylthiouracil (80 mg/Kg/day, n = 10), a cholesterol-rich diet plus methylthiouracil and rosiglitazone (4 mg/kg/day, n = 10). The rosiglitazone treatment was started from one month after the start of cholesterol-rich diet plus methylthiouracil, and lasted five months. Cultured vascular smooth muscle cells (VSMCs) were pretreated with 1 μmol/L angiotensin II (ANG II) for 6 h and randomly divided into the control group; the ANG II group (1 μmol/L ANG II); the groups respectively treated with different concentration rosiglitazone (20, 30, 50) μmol/L for 12 h; the groups treated with 30 μmol/L rosiglitazone for (6, 12, 24) h. Morphology changes of the aortic tissues were observed by hematoxylin and eosin stain. The VSMC growth was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Angiotensin II and expression of angiotensin receptors were determined by radioimmunoassay, reverse transcription polymerase chain reaction (RT-PCR), western blot, and immunohistochemistry.
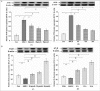
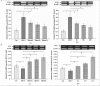
Results: After 6 months, lipid deposition, VSMC proliferation and migration toward intima were observed in aortic tissues in the rats on a cholesterol-rich diet plus methylthiouracil, while these pathological changes induced by the cholesterol-rich diet were significantly suppressed by rosiglitazone. In addition, VSMC proliferation induced by ANG II was markedly inhibited by rosiglitazone. Rosiglitazone markedly down-regulated expression of angiotensin type 1 receptor (AT1R) and up-regulated expression of angiotensin type 2 receptor (AT2R) in the aortic tissues and ANG II-treated VSMCs.
Conclusions: The present study demonstrated that PPARγ agonist rosiglitazone suppressed ANG II-induced VSMC proliferation in vitro and early atherosclerotic formation evoked by cholesterol-rich diet in vivo. These vasculoprotective effects of rosiglitazone were mediated at least partially by reduction in local tissue ANG II concentration, down-regulation of AT1R expression and up-regulation of AT2R expression both at the mRNA and protein levels.
Figures



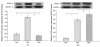
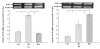
Similar articles
-
The inhibitory effects of rosiglitazone on cardiac hypertrophy through modulating the renin-angiotensin system in diet-induced hypercholesterolemic rats.Cell Biochem Funct. 2010 Jan;28(1):58-65. doi: 10.1002/cbf.1621. Cell Biochem Funct. 2010. PMID: 20029960
-
PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway.Lab Invest. 2009 Aug;89(8):887-902. doi: 10.1038/labinvest.2009.45. Epub 2009 May 18. Lab Invest. 2009. PMID: 19451898
-
Telmisartan downregulates angiotensin II type 1 receptor through activation of peroxisome proliferator-activated receptor gamma.Cardiovasc Res. 2006 Oct 1;72(1):184-90. doi: 10.1016/j.cardiores.2006.07.014. Epub 2006 Jul 21. Cardiovasc Res. 2006. PMID: 16938288
-
Regulation of vascular angiotensin II type 1 and type 2 receptor and angiotensin-(1-7)/MasR signaling in normal and hypertensive pregnancy.Biochem Pharmacol. 2024 Feb;220:115963. doi: 10.1016/j.bcp.2023.115963. Epub 2023 Dec 5. Biochem Pharmacol. 2024. PMID: 38061417 Free PMC article. Review.
-
Mitochondrial angiotensin receptors and cardioprotective pathways.Am J Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1426-H1438. doi: 10.1152/ajpheart.00772.2018. Epub 2019 Apr 12. Am J Physiol Heart Circ Physiol. 2019. PMID: 30978131 Free PMC article. Review.
Cited by
-
Effects of thiazolidinedione therapy on inflammatory markers of type 2 diabetes: a meta-analysis of randomized controlled trials.PLoS One. 2015 Apr 21;10(4):e0123703. doi: 10.1371/journal.pone.0123703. eCollection 2015. PLoS One. 2015. PMID: 25897968 Free PMC article.
-
Rosiglitazone attenuates renal injury caused by hyperlipidemic pancreatitis.Int J Clin Exp Pathol. 2015 May 1;8(5):4332-43. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191125 Free PMC article.
-
Short-Term Therapy with Rosiglitazone, a PPAR-γ Agonist, Improves Metabolic Profile and Vascular Function in Nonobese Lean Wistar Rats.ISRN Pharmacol. 2012;2012:130347. doi: 10.5402/2012/130347. Epub 2012 Aug 21. ISRN Pharmacol. 2012. PMID: 22957269 Free PMC article.
-
PPARs in atherosclerosis: The spatial and temporal features from mechanism to druggable targets.J Adv Res. 2025 Mar;69:225-244. doi: 10.1016/j.jare.2024.03.020. Epub 2024 Mar 29. J Adv Res. 2025. PMID: 38555000 Free PMC article. Review.
-
Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion.PLoS One. 2013 Apr 30;8(4):e62833. doi: 10.1371/journal.pone.0062833. Print 2013. PLoS One. 2013. PMID: 23646149 Free PMC article.
References
-
- Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. - PubMed
-
- Marx N, Kehrle B, Kohlhammer K, Grüb M, Koenig W, Hombach V, Libby P, Plutzky J. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–710. doi: 10.1161/01.RES.0000014225.20727.8F. - DOI - PMC - PubMed
-
- Shiomi T, Tsutsui H, Hayashidani S, Suematsu N, Ikeuchi M, Wen J, Ishibashi M, Kubota T, Egashira K, Takeshita A. Pioglitazone, a peroxisome proliferator-activated receptor-fgammag agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106:3126–3132. doi: 10.1161/01.CIR.0000039346.31538.2C. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

