Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells
- PMID: 21270052
- PMCID: PMC3035090
- DOI: 10.1242/dev.055236
Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells
Erratum in
- Development. 2011 Apr;138(7):1445
- Development. 2011 Mar;138(5).doi: 10.1242/dev.065904
Abstract
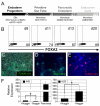
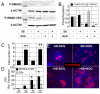
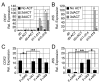
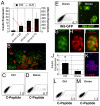
The generation of insulin-producing β-cells from human pluripotent stem cells is dependent on efficient endoderm induction and appropriate patterning and specification of this germ layer to a pancreatic fate. In this study, we elucidated the temporal requirements for TGFβ family members and canonical WNT signaling at these developmental stages and show that the duration of nodal/activin A signaling plays a pivotal role in establishing an appropriate definitive endoderm population for specification to the pancreatic lineage. WNT signaling was found to induce a posterior endoderm fate and at optimal concentrations enhanced the development of pancreatic lineage cells. Inhibition of the BMP signaling pathway at specific stages was essential for the generation of insulin-expressing cells and the extent of BMP inhibition required varied widely among the cell lines tested. Optimal stage-specific manipulation of these pathways resulted in a striking 250-fold increase in the levels of insulin expression and yielded populations containing up to 25% C-peptide+ cells.
Figures



References
-
- Ameri J., Stahlberg A., Pedersen J., Johansson J. K., Johannesson M. M., Artner I., Semb H. (2010). FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells 28, 45-56 - PubMed
-
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400, 877-881 - PubMed
-
- Cai J., Yu C., Liu Y., Chen S., Guo Y., Yong J., Lu W., Ding M., Deng H. (2009). Generation of homogeneous PDX1(+) pancreatic progenitors from human ES cell-derived endoderm cells. J. Mol. Cell Biol. 2, 50-60 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

