The absolute threshold of cone vision
- PMID: 21270115
- PMCID: PMC3671617
- DOI: 10.1167/11.1.21
The absolute threshold of cone vision
Abstract
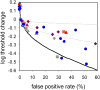
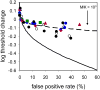
We report measurements of the absolute threshold of cone vision, which has been previously underestimated due to suboptimal conditions or overly strict subjective response criteria. We avoided these limitations by using optimized stimuli and experimental conditions while having subjects respond within a rating scale framework. Small (1' fwhm), brief (34 ms), monochromatic (550 nm) stimuli were foveally presented at multiple intensities in dark-adapted retina for 5 subjects. For comparison, 4 subjects underwent similar testing with rod-optimized stimuli. Cone absolute threshold, that is, the minimum light energy for which subjects were just able to detect a visual stimulus with any response criterion, was 203 ± 38 photons at the cornea, ~0.47 log unit lower than previously reported. Two-alternative forced-choice measurements in a subset of subjects yielded consistent results. Cone thresholds were less responsive to criterion changes than rod thresholds, suggesting a limit to the stimulus information recoverable from the cone mosaic in addition to the limit imposed by Poisson noise. Results were consistent with expectations for detection in the face of stimulus uncertainty. We discuss implications of these findings for modeling the first stages of human cone vision and interpreting psychophysical data acquired with adaptive optics at the spatial scale of the receptor mosaic.
Figures








References
-
- Arathorn DW, Yang Q, Vogel CR, Zhang Y, Tiruveedhula P, Roorda A. Retinally stabilized cone-targeted stimulus delivery. Optics Express. 2007;15(21):13731–13744. - PubMed
-
- Barlow HB. Retinal noise and absolute threshold. Journal of the Optical Society of America. 1956;46(8):634–639. - PubMed
-
- Barlow HB. Visual Problems of Colour. II. London: Her Majesty's Stationery Office; 1958. Intrinsic noise of cones; pp. 617–630.
-
- Barlow HB. Retinal and central factors in human vision limited by noise. In: Barlow HB, Fatt P, editors. Vertebrate Photoreception. San Fransisco, California: Academic Press; 1977.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

