Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease
- PMID: 21270160
- PMCID: PMC3067869
- DOI: 10.1128/JVI.02521-10
Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease
Abstract
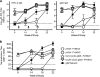
To date, no vaccine that is safe and effective against herpes simplex virus 2 (HSV-2) disease has been licensed. In this study, we evaluated a DNA prime-formalin-inactivated-HSV-2 (FI-HSV2) boost vaccine approach in the guinea pig model of acute and recurrent HSV-2 genital disease. Five groups of guinea pigs were immunized and intravaginally challenged with HSV-2. Two groups were primed with plasmid DNAs encoding the secreted form of glycoprotein D2 (gD2t) together with two genes required for viral replication, either the helicase (UL5) and DNA polymerase (UL30) genes or the single-stranded DNA binding protein (UL29) and primase (UL52) genes. Both DNA-primed groups were boosted with FI-HSV2 formulated with monophosphoryl lipid A (MPL) and alum adjuvants. Two additional groups were primed with the empty backbone plasmid DNA (pVAX). These two groups were boosted with MPL and alum (MPL-alum) together with either formalin-inactivated mock HSV-2 (FI-Mock) or with FI-HSV2. The final group was immunized with gD2t protein in MPL-alum. After challenge, 0/9 animals in the group primed with UL5, UL30, and gD2t DNAs and all 10 animals in the mock-immunized control group (pVAX-FI-Mock) developed primary lesions. All mock controls developed recurrent lesions through day 100 postchallenge. Only 1 guinea pig in the group primed with pVAX DNA and boosted with FI-HSV2 (pVAX-FI-HSV2 group) and 2 guinea pigs in the group primed with UL5, UL30, and gD2t DNAs and boosted with FI-HSV2 (UL5, UL30, gD2t DNA-FI-HSV2 group) developed recurrent lesions. Strikingly, the UL5, UL30, gD2t DNA-FI-HSV2 group showed a 97% reduction in recurrent lesion days compared with the mock controls, had the highest reduction in days with recurrent disease, and contained the lowest mean HSV-2 DNA load in the dorsal root ganglia.
Figures




Similar articles
-
Inactivated HSV-2 in MPL/alum adjuvant provides nearly complete protection against genital infection and shedding following long term challenge and rechallenge.Vaccine. 2012 Oct 12;30(46):6541-6550. doi: 10.1016/j.vaccine.2012.08.049. Epub 2012 Sep 1. Vaccine. 2012. PMID: 22947141 Free PMC article.
-
Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge.J Virol. 2015 Jan;89(1):83-96. doi: 10.1128/JVI.02380-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320297 Free PMC article.
-
Single and combination herpes simplex virus type 2 glycoprotein vaccines adjuvanted with CpG oligodeoxynucleotides or monophosphoryl lipid A exhibit differential immunity that is not correlated to protection in animal models.Clin Vaccine Immunol. 2011 Oct;18(10):1702-9. doi: 10.1128/CVI.05071-11. Epub 2011 Aug 18. Clin Vaccine Immunol. 2011. PMID: 21852545 Free PMC article.
-
Glycoprotein D adjuvant herpes simplex virus vaccine.Expert Rev Vaccines. 2005 Oct;4(5):615-27. doi: 10.1586/14760584.4.5.615. Expert Rev Vaccines. 2005. PMID: 16221064 Review.
-
Improving immunogenicity and efficacy of vaccines for genital herpes containing herpes simplex virus glycoprotein D.Expert Rev Vaccines. 2014 Dec;13(12):1475-88. doi: 10.1586/14760584.2014.951336. Epub 2014 Aug 20. Expert Rev Vaccines. 2014. PMID: 25138572 Review.
Cited by
-
Comparison of the host immune response to herpes simplex virus 1 (HSV-1) and HSV-2 at two different mucosal sites.J Virol. 2012 Jul;86(13):7454-8. doi: 10.1128/JVI.00702-12. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532684 Free PMC article.
-
Herpes Simplex Vaccines: Prospects of Live-attenuated HSV Vaccines to Combat Genital and Ocular infections.Curr Clin Microbiol Rep. 2015 Sep 1;2(3):125-136. doi: 10.1007/s40588-015-0020-4. Epub 2015 Jul 1. Curr Clin Microbiol Rep. 2015. PMID: 27114893 Free PMC article.
-
HSV-2: in pursuit of a vaccine.J Clin Invest. 2011 Dec;121(12):4600-9. doi: 10.1172/JCI57148. Epub 2011 Dec 1. J Clin Invest. 2011. PMID: 22133885 Free PMC article. Review.
-
The immunologic basis for severe neonatal herpes disease and potential strategies for therapeutic intervention.Clin Dev Immunol. 2013;2013:369172. doi: 10.1155/2013/369172. Epub 2013 Mar 31. Clin Dev Immunol. 2013. PMID: 23606868 Free PMC article. Review.
-
An Adenovirus-Based Recombinant Herpes Simplex Virus 2 (HSV-2) Therapeutic Vaccine Is Highly Protective against Acute and Recurrent HSV-2 Disease in a Guinea Pig Model.Viruses. 2023 Jan 13;15(1):219. doi: 10.3390/v15010219. Viruses. 2023. PMID: 36680259 Free PMC article.
References
-
- Baldridge, J. R., and R. T. Crane. 1999. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods 19:103-107. - PubMed
-
- Centers for Disease Control and Prevention. 2010. Seroprevalence of herpes simplex virus type 2 among persons aged 14-49 years—United States, 2005-2008. MMWR Morb. Mortal. Wkly Rep. 59:456-459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

