The adipocyte-expressed forkhead transcription factor Foxc2 regulates metabolism through altered mitochondrial function
- PMID: 21270254
- PMCID: PMC3028341
- DOI: 10.2337/db10-0409
The adipocyte-expressed forkhead transcription factor Foxc2 regulates metabolism through altered mitochondrial function
Abstract
Objective: Previous findings demonstrate that enhanced expression of the forkhead transcription factor Foxc2 in adipose tissue leads to a lean and insulin-sensitive phenotype. These findings prompted us to further investigate the role of Foxc2 in the regulation of genes of fundamental importance for metabolism and mitochondrial function.
Research design and methods: The effects of Foxc2 on expression of genes involved in mitochondriogenesis and mitochondrial function were assessed by quantitative real-time PCR. The potential of a direct transcriptional regulation of regulated genes was tested in promoter assays, and mitochondrial morphology was investigated by electron microscopy. Mitochondrial function was tested by measuring oxygen consumption and extracellular acidification rates as well as palmitate oxidation.
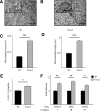
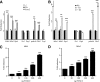
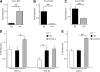
Results: Enhanced expression of FOXC2 in adipocytes or in cells with no endogenous Foxc2 expression induces mitochondriogenesis and an elongated mitochondrial morphology. Together with increased aerobic metabolic capacity, increased palmitate oxidation, and upregulation of genes encoding respiratory complexes and of brown fat-related genes, Foxc2 also specifically induces mitochondrial fusion genes in adipocytes. Among tested forkhead genes, Foxc2 is unique in its ability to trans-activate the nuclear-encoded mitochondrial transcription factor A (mtTFA/Tfam) gene--a master regulator of mitochondrial biogenesis. In human adipose tissue the expression levels of mtTFA/Tfam and of fusion genes also correlate with that of Foxc2.
Conclusions: We previously showed that a high-calorie diet and insulin induce Foxc2 in adipocytes; the current findings identify a previously unknown role for Foxc2 as an important metabo-regulator of mitochondrial morphology and metabolism.
Figures




References
-
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005;434:113–118 - PubMed
-
- Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 - PubMed
-
- Björntorp P, Scherstén T, Fagerberg SE. Respiration and phosphorylation of mitochondria isolated from the skeletal muscle of diabetic and normal subjects. Diabetologia 1967;3:346–352 - PubMed
-
- Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

