The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis
- PMID: 21270439
- PMCID: PMC3057701
- DOI: 10.1091/mbc.E10-08-0671
The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis
Abstract
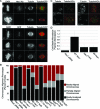
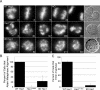
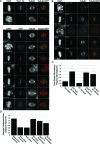
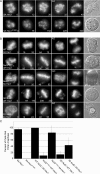
Successful mitosis requires that kinetochores stably attach to the plus ends of spindle microtubules. Central to generating these attachments is the NDC80 complex, made of the four proteins Spc24, Spc25, Nuf2, and Hec1/Ndc80. Structural studies have revealed that portions of both Hec1 and Nuf2 N termini fold into calponin homology (CH) domains, which are known to mediate microtubule binding in certain proteins. Hec1 also contains a basic, positively charged stretch of amino acids that precedes its CH domain, referred to as the "tail." Here, using a gene silence and rescue approach in HeLa cells, we show that the CH domain of Hec1, the CH domain of Nuf2, and the Hec1 tail each contributes to kinetochore-microtubule attachment in distinct ways. The most severe defects in kinetochore-microtubule attachment were observed in cells rescued with a Hec1 CH domain mutant, followed by those rescued with a Hec1 tail domain mutant. Cells rescued with Nuf2 CH domain mutants, however, generated stable kinetochore-microtubule attachments but failed to generate wild-type interkinetochore tension and failed to enter anaphase in a timely manner. These data suggest that the CH and tail domains of Hec1 generate essential contacts between kinetochores and microtubules in cells, whereas the Nuf2 CH domain does not.
Figures


References
-
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

