Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse
- PMID: 21270442
- PMCID: PMC3069671
- DOI: 10.1182/blood-2010-08-301796
Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse
Abstract
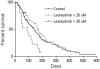
In a randomized trial of therapy for FMS-like tyrosine kinase-3 (FLT3) mutant acute myeloid leukemia in first relapse, 224 patients received chemotherapy alone or followed by 80 mg of the FLT3 inhibitor lestaurtinib twice daily. Endpoints included complete remission or complete remission with incomplete platelet recovery (CR/CRp), overall survival, safety, and tolerability. Correlative studies included pharmacokinetics and analysis of in vivo FLT3 inhibition. There were 29 patients with CR/CRp in the lestaurtinib arm and 23 in the control arm (26% vs 21%; P = .35), and no difference in overall survival between the 2 arms. There was evidence of toxicity in the lestaurtinib-treated patients, particularly those with plasma levels in excess of 20 μM. In the lestaurtinib arm, FLT3 inhibition was highly correlated with remission rate, but target inhibition on day 15 was achieved in only 58% of patients receiving lestaurtinib. Given that such a small proportion of patients on this trial achieved sustained FLT3 inhibition in vivo, any conclusions regarding the efficacy of combining FLT3 inhibition with chemotherapy are limited. Overall, lestaurtinib treatment after chemotherapy did not increase response rates or prolong survival of patients with FLT3 mutant acute myeloid leukemia in first relapse. This study is registered at www.clinicaltrials.gov as #NCT00079482.
Figures




Comment in
-
Rethinking bioactivity of FLT3 inhibitors.Blood. 2011 Mar 24;117(12):3247-8. doi: 10.1182/blood-2011-02-334540. Blood. 2011. PMID: 21436074 No abstract available.
References
-
- Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918. - PubMed
-
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. - PubMed
-
- Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93(9):3074–3080. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. - PubMed
-
- Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

