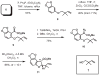
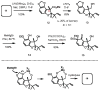
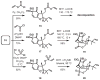
Studies toward the synthesis of maoecrystal V
- PMID: 21271687
- PMCID: PMC3373731
- DOI: 10.1021/ol1031238
Studies toward the synthesis of maoecrystal V
Abstract
An approach toward the synthesis of maoecrystal V is described. The synthetic strategy for this approach was designed to address unique challenges posed by the strained tetrahydrofuran ring at the center of the target structure.
Figures
Similar articles
-
Total synthesis of the Isodon diterpene sculponeatin N.Angew Chem Int Ed Engl. 2014 Mar 10;53(11):2988-91. doi: 10.1002/anie.201310060. Epub 2014 Feb 12. Angew Chem Int Ed Engl. 2014. PMID: 24519748
-
Synthetic study toward the total synthesis of maoecrystal V.Org Lett. 2009 Nov 5;11(21):4770-3. doi: 10.1021/ol9014392. Org Lett. 2009. PMID: 19863143
-
A synthesis of the carbon skeleton of maoecrystal V.Org Lett. 2009 Nov 5;11(21):4774-6. doi: 10.1021/ol901963v. Org Lett. 2009. PMID: 19795876 Free PMC article.
-
The total synthesis of Isodon diterpenes.Angew Chem Int Ed Engl. 2014 Sep 26;53(40):10588-99. doi: 10.1002/anie.201404482. Epub 2014 Aug 27. Angew Chem Int Ed Engl. 2014. PMID: 25159338 Review.
-
Synthetic efforts toward cyathane diterpenoid natural products.Nat Prod Rep. 2009 May;26(5):661-80. doi: 10.1039/b811227b. Epub 2009 Mar 16. Nat Prod Rep. 2009. PMID: 19387500 Free PMC article. Review.
Cited by
-
Total synthesis of maoecrystal V: early-stage C-H functionalization and lactone assembly by radical cyclization.J Am Chem Soc. 2013 Oct 2;135(39):14552-5. doi: 10.1021/ja408231t. Epub 2013 Sep 23. J Am Chem Soc. 2013. PMID: 24047444 Free PMC article.
-
Development of an enolate alkynylation approach towards the synthesis of the taiwanschirin natural products.Chem Sci. 2021 Sep 20;12(40):13392-13397. doi: 10.1039/d1sc04247e. eCollection 2021 Oct 20. Chem Sci. 2021. PMID: 34777757 Free PMC article.
-
Total synthesis of (±)-maoecrystal V.J Am Chem Soc. 2012 Nov 14;134(45):18860-7. doi: 10.1021/ja309905j. Epub 2012 Nov 5. J Am Chem Soc. 2012. PMID: 23126440 Free PMC article.
-
Oxidative Coupling of Enolates, Enol Silanes and Enamines: Methods and Natural Product Synthesis.European J Org Chem. 2012 Sep 1;2012(26):4881-4896. doi: 10.1002/ejoc.201200665. Epub 2012 Jul 16. European J Org Chem. 2012. PMID: 23471479 Free PMC article.
-
Evaluation of 'east-to-west' ether-forming strategies for the total synthesis of maoecrystal V.Tetrahedron Lett. 2013 Feb 13;54(7):635-637. doi: 10.1016/j.tetlet.2012.11.135. Tetrahedron Lett. 2013. PMID: 23316095 Free PMC article.
References
-
- Li S-H, Wang J, Niu X-M, Shen Y-H, Zhang H-J, Sun H-D, Li M-L, Tian Q-E, Lu Y, Cao P, Zheng Q-T. Org Lett. 2004;6:4327–4330. - PubMed
-
- Gong J, Lin G, Li C-C, Yang Z. Org Lett. 2009;11:4770–4773. - PubMed
- Krawczuk PJ, Schöne N, Baran PS. Org Lett. 2009;11:4774–4776. - PMC - PubMed
- Peng F, Yu M, Danishefsky SJ. Tetrahedron Lett. 2009;50:6586–6587. - PMC - PubMed
- Nicolaou KC, Dong L, Deng L, Talbot AC, Chen DY-K. Chem Commun. 2010;46:70–72. - PubMed
- Lazarski KE, Hu DX, Stern CL, Thomson RJ. Org Lett. 2010;12:3010–3013. - PMC - PubMed
- Baitinger I, Mayer P, Trauner D. Org Lett. 2010;12:5656–5659. - PubMed
-
- Gong J, Lin G, Sun W, Li C-C, Yang Z. J Am Chem Soc. 2010;132:16745–16746. - PubMed
-
-
For recent examples using masked ortho-quinone as substrates for Diels–Alder reactions, see: Chu C-S, Lee T-H, Rao PD, Song L-D, Liao C-C. J Org Chem. 1999;64:4111–4118.Nicolaou KC, Toh Q-Y, Chen DY-K. J Am Chem Soc. 2008;130:11292–11293.Singh V, Bhalerao P, Mobin SM. Tetrahedron Lett. 2010;51:3337–3339.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources