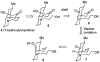
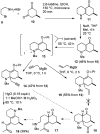
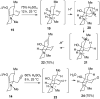
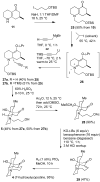
Synthesis of (±)-7-hydroxylycopodine
- PMID: 21271726
- PMCID: PMC3056437
- DOI: 10.1021/ol200119h
Synthesis of (±)-7-hydroxylycopodine
Abstract
A six step synthesis of (±)-7-hydroxylycopodine has been achieved in 5% overall yield. In the key step, a Prins cyclization of a bicyclic keto alkyne in 60% H(2)SO(4) forms a tricyclic dihydroxy amino ketone.
Figures
Similar articles
-
Synthesis of (±)-7-hydroxylycopodine.J Org Chem. 2012 Sep 7;77(17):7143-56. doi: 10.1021/jo300353t. Epub 2012 Mar 30. J Org Chem. 2012. PMID: 22443298 Free PMC article.
-
Toward a unified approach for the lycopodines: synthesis of 10-hydroxylycopodine, deacetylpaniculine, and paniculine.Org Lett. 2013 Feb 15;15(4):736-9. doi: 10.1021/ol303272w. Epub 2013 Feb 5. Org Lett. 2013. PMID: 23384410 Free PMC article.
-
Total synthesis of (+)-epiquinamide.Org Lett. 2005 Sep 1;7(18):4005-7. doi: 10.1021/ol0515715. Org Lett. 2005. PMID: 16119953
-
Indolizidine and quinolizidine alkaloids.Nat Prod Rep. 1990 Dec;7(6):485-513. doi: 10.1039/np9900700485. Nat Prod Rep. 1990. PMID: 2084595 Review. No abstract available.
-
Indolizidine and quinolizidine alkaloids.Nat Prod Rep. 1997 Dec;14(6):619-36. doi: 10.1039/np9971400619. Nat Prod Rep. 1997. PMID: 9418297 Review. No abstract available.
Cited by
-
Bi(OTf)3-, TfOH-, and TMSOTf-mediated, one-pot epoxide rearrangement, addition, and intramolecular silyl-modified Sakurai (ISMS) cascade toward dihydropyrans: comparison of catalysts and role of Bi(OTf)3.J Org Chem. 2011 Nov 18;76(22):9269-77. doi: 10.1021/jo201478d. Epub 2011 Oct 25. J Org Chem. 2011. PMID: 21916500 Free PMC article.
-
Synthesis of (±)-7-hydroxylycopodine.J Org Chem. 2012 Sep 7;77(17):7143-56. doi: 10.1021/jo300353t. Epub 2012 Mar 30. J Org Chem. 2012. PMID: 22443298 Free PMC article.
-
Toward a unified approach for the lycopodines: synthesis of 10-hydroxylycopodine, deacetylpaniculine, and paniculine.Org Lett. 2013 Feb 15;15(4):736-9. doi: 10.1021/ol303272w. Epub 2013 Feb 5. Org Lett. 2013. PMID: 23384410 Free PMC article.
References
-
- Ortega MG, Agnese AM, Cabrera JL. Tetrahedron Lett. 2004;45:7003–7005.
- Vallejo MG, Ortega MG, Cabrera JL, Carlini VP, Rubiales de Barioglio S, Almiron RS, Ramirez OA, Agnese AM. J Nat Prod. 2009;72:156–158. - PubMed
-
note that the methyl group of sauroine is attached to the wrong carbon.
-
-
For recent reviews on lycopodium alkaloids, see: Kobayashi J, Morita H. In: The Alkaloids: Chemistry and Biology. Cordell GA, editor. Vol. 61. Elsevier; San Diego, CA: 2005. pp. 1–57.Ma X, Gang DR. Nat Prod Rep. 2004;21:752–772.Hirasawa Y, Kobayashi J, Morita H. Heterocycles. 2009;77:679–729.
-
-
-
For recent reviews on huperzine A for treatment of Alzheimer's disease, see: Hostettmann K, Borloz A, Urbain A, Marston A. Current Org Chem. 2006;10:825–847.Zhu DY, Tan CH, Li YM. In: Medicinal Chemistry of Bioactive Natural Products. Liang XT, Fang WS, editors. WILEY-VCH; New York: 2006. pp. 143–182.Bai D. Pure Appl Chem. 2007;79:469–479.Little JT, Walsh S, Aisen PS. Expert Opinion on Invest Drugs. 2008;17:209–215.Desilets AR, Gickas JJ, Dunican KC. Annals Pharmacother. 2009;43:514–518.
-
-
-
Stork G, Kretchmer RA, Schlessinger RH. J Am Chem Soc. 1968;90:1647–1648.Ayer WA, Bowman WR, Joseph TC, Smith P. J Am Chem Soc. 1968;90:1648–1650.Kim S, Bando Y, Horii Z. Tetrahedron Lett. 1978:2293–2294.Heathcock CH, Kleinman EF, Binkley ES. J Am Chem Soc. 1982;104:1054–1068.Schumann D, Müller HJ, Naumann A. Liebigs Ann Chem. 1982:1700–1705.Kraus GA, Hon YS. J Am Chem Soc. 1985;107:4341–4342. and Heterocycles. 1987;25:377–386.Padwa A, Brodney MA, Marino JP, Sheehan SM. J Org Chem. 1997;62:78–87.Greico PA, Dai YJ. J Am Chem Soc. 1998;120:5128–5129.Mori M, Hori K, Akashi M, Hori M, Sato Y, Nishida M. Angew Chem, Int Ed. 1998;37:636–637.Yang H, Carter RG, Zakharov LN. J Am Chem Soc. 2008;130:9238–9239.Yang H, Carter RG. J Org Chem. 2010;75:4929–4938. (l) For a review of lycopodine syntheses, see: Hudlický T, Reed JW. The Way of Synthesis. Wiley-VCH; Weinheim: 2007. pp. 573–602.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources