Inflammatory and cytotoxic responses of an alveolar-capillary coculture model to silica nanoparticles: comparison with conventional monocultures
- PMID: 21272353
- PMCID: PMC3040689
- DOI: 10.1186/1743-8977-8-6
Inflammatory and cytotoxic responses of an alveolar-capillary coculture model to silica nanoparticles: comparison with conventional monocultures
Abstract
Background: To date silica nanoparticles (SNPs) play an important role in modern technology and nanomedicine. SNPs are present in various materials (tyres, electrical and thermal insulation material, photovoltaic facilities). They are also used in products that are directly exposed to humans such as cosmetics or toothpaste. For that reason it is of great concern to evaluate the possible hazards of these engineered particles for human health. Attention should primarily be focussed on SNP effects on biological barriers. Accidentally released SNP could, for example, encounter the alveolar-capillary barrier by inhalation. In this study we examined the inflammatory and cytotoxic responses of monodisperse amorphous silica nanoparticles (aSNPs) of 30 nm in size on an in vitro coculture model mimicking the alveolar-capillary barrier and compared these to conventional monocultures.
Methods: Thus, the epithelial cell line, H441, and the endothelial cell line, ISO-HAS-1, were used in monoculture and in coculture on opposite sides of a filter membrane. Cytotoxicity was evaluated by the MTS assay, detection of membrane integrity (LDH release), and TER (Transepithelial Electrical Resistance) measurement. Additionally, parameters of inflammation (sICAM-1, IL-6 and IL-8 release) and apoptosis markers were investigated.
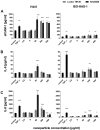
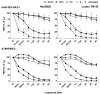
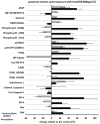
Results: Regarding toxic effects (viability, membrane integrity, TER) the coculture model was less sensitive to apical aSNP exposure than the conventional monocultures of the appropriate cells. On the other hand, the in vitro coculture model responded with the release of inflammatory markers in a much more sensitive fashion than the conventional monoculture. At concentrations that were 10-100fold less than the toxic concentrations the apically exposed coculture showed a release of IL-6 and IL-8 to the basolateral side. This may mimic the early inflammatory events that take place in the pulmonary alveoli after aSNP inhalation. Furthermore, a number of apoptosis markers belonging to the intrinsic pathway were upregulated in the coculture following aSNP treatment. Analysis of the individual markers indicated that the cells suffered from DNA damage, hypoxia and ER-stress.
Conclusion: We present evidence that our in vitro coculture model of the alveolar-capillary barrier is clearly advantageous compared to conventional monocultures in evaluating the extent of damage caused by hazardous material encountering the principle biological barrier in the lower respiratory tract.
Figures
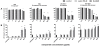



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

