Liver kinase B1 (LKB1) in the pathogenesis of UVB-induced murine basal cell carcinoma
- PMID: 21272562
- PMCID: PMC3072754
- DOI: 10.1016/j.abb.2011.01.006
Liver kinase B1 (LKB1) in the pathogenesis of UVB-induced murine basal cell carcinoma
Abstract
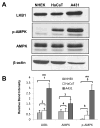
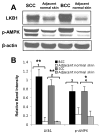
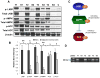
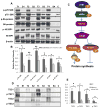
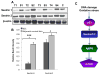
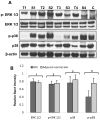
LKB1, a known tumor suppressor, is mutated in Peutz-Jeghers Syndrome (PJS). It is responsible for the enhanced cancer risk in patients with PJS. Dysregulation of LKB1-dependent signaling also occurs in various epithelial cancers. UVB alters the expression of LKB1, though its role in the pathogenesis of skin cancer is unknown. Here we describe upregulation of LKB1 expression in UVB-induced murine basal cell carcinoma (BCC) and in human skin tumor keratinocytes. AMP-kinase and acetyl Co-A carboxylase, the downstream LKB1 targets, are also enhanced in this neoplasm. In addition, p-Akt, a kinase which inactivates GSK3β by its phosphorylation, is enhanced in BCCs. Consistently, an accumulation of p-GSK3β and an increase in activated nuclear β-catenin are found. mTOR signaling, which is also inhibited by LKB1, remains upregulated in BCCs. However, a marked decrease in the expression of sestrins, which function as potent negative regulators of mTOR is observed. Metformin, a known chemical inducer of this pathway, was found effective in immortalized HaCaT keratinocytes, but failed to activate the LKB1-dependent signaling in human carcinoma A431 cells. Thus, our data show that the LKB1/AMPK axis fails to regulate mTOR pathway, and a complex regulatory mechanism exists for the persistent mTOR activation in murine BCCs.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis.Breast Cancer Res. 2012 Feb 21;14(1):R35. doi: 10.1186/bcr3128. Breast Cancer Res. 2012. PMID: 22353783 Free PMC article.
-
Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis.Hum Mol Genet. 2012 Oct 15;21(20):4394-405. doi: 10.1093/hmg/dds272. Epub 2012 Jul 12. Hum Mol Genet. 2012. PMID: 22791749 Free PMC article.
-
AMPK/TSC2/mTOR-signaling intermediates are not necessary for LKB1-mediated nuclear retention of PTEN tumor suppressor.Neuro Oncol. 2011 Feb;13(2):184-94. doi: 10.1093/neuonc/noq163. Epub 2010 Dec 1. Neuro Oncol. 2011. PMID: 21123367 Free PMC article.
-
UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer.Adv Exp Med Biol. 2017;996:27-40. doi: 10.1007/978-3-319-56017-5_3. Adv Exp Med Biol. 2017. PMID: 29124688 Review.
-
Targeting LKB1 signaling in cancer.Biochim Biophys Acta. 2013 Apr;1835(2):194-210. doi: 10.1016/j.bbcan.2012.12.006. Epub 2012 Dec 31. Biochim Biophys Acta. 2013. PMID: 23287572 Review.
Cited by
-
Metformin inhibits the proliferation of A431 cells by modulating the PI3K/Akt signaling pathway.Exp Ther Med. 2015 Apr;9(4):1401-1406. doi: 10.3892/etm.2015.2220. Epub 2015 Jan 27. Exp Ther Med. 2015. PMID: 25780442 Free PMC article.
-
Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs.Aging (Albany NY). 2012 Jul;4(7):480-98. doi: 10.18632/aging.100472. Aging (Albany NY). 2012. PMID: 22837425 Free PMC article.
-
GSK3β signaling is involved in ultraviolet B-induced activation of autophagy in epidermal cells.Int J Oncol. 2012 Nov;41(5):1782-8. doi: 10.3892/ijo.2012.1620. Epub 2012 Sep 5. Int J Oncol. 2012. PMID: 22961228 Free PMC article.
-
Targeting the AMP-Activated Protein Kinase for Cancer Prevention and Therapy.Front Oncol. 2013 Jul 15;3:175. doi: 10.3389/fonc.2013.00175. eCollection 2013. Front Oncol. 2013. PMID: 23875169 Free PMC article.
-
LKB1 suppresses androgen synthesis in a mouse model of hyperandrogenism via IGF-1 signaling.FEBS Open Bio. 2019 Oct;9(10):1817-1825. doi: 10.1002/2211-5463.12723. Epub 2019 Sep 12. FEBS Open Bio. 2019. Retraction in: FEBS Open Bio. 2025 Mar;15(3):525. doi: 10.1002/2211-5463.13948. PMID: 31433577 Free PMC article. Retracted.
References
-
- Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, Roenigk RK. Incidence of Basal Cell and Squamous Cell Carcinoma in a population younger than 40 years. JAMA. 2005;294:681–690. - PubMed
-
- Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. British Journal of Dermatology. 2008;159:35–48. - PubMed
-
- Richmond-Sinclair NM, Pandeya N, Ware RS, Neal RE, Williams GM, van der Pols JC, Green AC. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. Journal of Investigative Dermatology. 2009;129:323–328. - PubMed
-
- Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Jr, Scott MP. Basal cell carcinoma in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. - PubMed
-
- Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCC and trichoblastomas in patched heterozygous knockout mice. Nature. 1999;11:1285–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

