Further evidence of olfactory ensheathing glia facilitating axonal regeneration after a complete spinal cord transection
- PMID: 21272578
- PMCID: PMC3085566
- DOI: 10.1016/j.expneurol.2011.01.007
Further evidence of olfactory ensheathing glia facilitating axonal regeneration after a complete spinal cord transection
Abstract
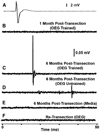
Spinal Wistar Hannover rats injected with olfactory ensheathing glia (OEG) have been shown to recover some bipedal stepping and climbing abilities. Given the intrinsic ability of the spinal cord to regain stepping with pharmacological agents or epidural stimulation after a complete mid-thoracic transection, we asked if functional recovery after OEG injections is due to changes in the caudal stump or facilitation of functional regeneration of axons across the transection site. OEG were injected rostral and caudal to the transection site immediately after transection. Robotically assisted step training in the presence of intrathecal injections of a 5-HT(2A) receptor agonist (quipazine) was used to facilitate recovery of stepping. Bipedal stepping as well as climbing abilities were tested over a 6-month period post-transection to determine any improvement in hindlimb functional due to OEG injections and/or step training. The ability for OEG to facilitate regeneration was analyzed electrophysiologically by transcranially stimulating the brainstem and recording motor evoked potentials (MEP) with chronically implanted intramuscular EMG electrodes in the soleus and tibalis anterior with and without intrathecal injections of noradrenergic, serotonergic, and glycinergic receptor antagonists. Analyses confirmed that along with improved stepping ability and increased use of the hindlimbs during climbing, only OEG rats showed recovery of MEP. In addition the MEP signals were eliminated after a re-transection of the spinal cord rostral to the original transection and were modified in the presence of receptor antagonists. These data indicate that improved hindlimb function after a complete transection was coupled with OEG-facilitated functional regeneration of axons. This article is part of a Special Issue entitled: Understanding olfactory ensheathing glia and their prospect for nervous system repair.
Published by Elsevier Inc.
Figures






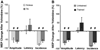

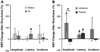

References
-
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010;16:302–307. - PubMed
-
- de Leon RD, Acosta CN. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J. Neurotrama. 2006;23:1147–1163. - PubMed
-
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J. Neurophysiol. 1999;82:359–369. - PubMed
-
- Edgerton VR, Roy RR. Spasticity: a switch from inhibition to excitation. Nat. Med. 2010;16:270–271. - PubMed

