The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function
- PMID: 21273243
- PMCID: PMC3096306
- DOI: 10.1093/cvr/cvr025
The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function
Abstract
Aims: In heart failure (HF), abnormal myocyte Ca(2+) handling has been implicated in cardiac arrhythmias and contractile dysfunction. In the present study, we investigated the relationships between Ca(2+) handling, reduced myocyte contractility, and enhanced arrhythmogenesis during HF progression in a canine model of non-ischaemic HF.
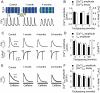
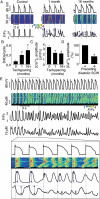
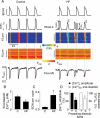
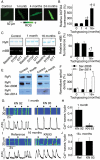
Methods and results: Key Ca(2+) handling parameters were determined by measuring cytosolic and intra-sarcoplasmic reticulum (SR) [Ca(2+)] in isolated ventricular myocytes at different stages of HF. The progression of HF was associated with an early and continuous increase in ryanodine receptor (RyR2)-mediated SR Ca(2+) leak. The increase in RyR2 activity was paralleled by an increase in the frequency of diastolic spontaneous Ca(2+) waves (SCWs) in HF myocytes under conditions of β-adrenergic stimulation. In addition to causing arrhythmogenic-delayed afterdepolarizations, SCWs decreased the amplitude of subsequent electrically evoked Ca(2+) transients by depleting SR Ca(2+). At late stages of HF, Ca(2+) release oscillated essentially independent of electrical pacing. The increased propensity for the generation of SCWs in HF myocytes was attributable to reduced ability of the RyR2 channels to become refractory following Ca(2+) release. The progressive alterations in RyR2 function and Ca(2+) cycling in HF myocytes were associated with sequential modifications of RyR2 by CaMKII-dependent phosphorylation and thiol oxidation.
Conclusion: These findings suggest that destabilized RyR2 activity due to excessive CaMKII phopshorylation and oxidation resulting in impaired post-release refractoriness is a common mechanism involved in arrhythmogenesis and contractile dysfunction in the failing heart.
Figures


Comment in
-
Leaky ryanodine receptors in the failing heart: the root of all evil?Cardiovasc Res. 2011 Jun 1;90(3):399-401. doi: 10.1093/cvr/cvr086. Epub 2011 Mar 29. Cardiovasc Res. 2011. PMID: 21447705 No abstract available.
References
-
- Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007;116:392–398. doi:10.1161/CIRCULATIONAHA.106.687103. - DOI - PubMed
-
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi:10.1093/cvr/cvn133. - DOI - PMC - PubMed
-
- Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. - PubMed
-
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi:10.1016/j.tcm.2003.12.002. - DOI - PubMed
-
- Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi:10.1152/physrev.00011.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

