Cc2d1a, a C2 domain containing protein linked to nonsyndromic mental retardation, controls functional maturation of central synapses
- PMID: 21273312
- PMCID: PMC3075281
- DOI: 10.1152/jn.00950.2010
Cc2d1a, a C2 domain containing protein linked to nonsyndromic mental retardation, controls functional maturation of central synapses
Abstract
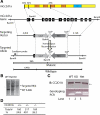
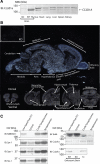
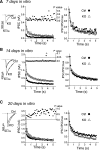
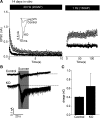
Cc2d1a is an evolutionarily conserved protein composed of NH(2)-terminal Drosophila melanogaster 14 domain (DM14) domains and a COOH-terminal C2 domain. Human patients with homozygotic mutation in the gene suffer from nonsyndromic mental retardation, implying that Cc2d1a functions in the central nervous system. To examine the physiological role of the Cc2d1a, we generated and analyzed Cc2d1a knockout (KO) mice. Cc2d1a KO mice die soon after birth, apparently because of their inability to breathe. Histological analysis of Cc2d1a KO animals did not identify any structural defects in the peripheral respiratory apparatus. However, functional analysis of synapses formed between Cc2d1a-deficient cortical neurons revealed a robust increase in the pace of maturation of evoked synaptic responses as well as synaptic vesicle trafficking. This synaptic anomaly was rescued by reintroducing full-length Cc2d1a but not C2-domain-deletion mutant, underscoring the functional importance of C2 domain. Our data suggest that Cc2d1a is required for mouse survival and performs essential function in controlling functional maturation of synapses.
Figures



References
-
- Agrawal N, Kango M, Mishra A, Sinha P. Neoplastic transformation, and aberrant cell-cell interactions in genetic mosaics of lethal(2)giant larvae (lgl), a tumor suppressor gene of Drosophila. Dev Biol 172: 218–229, 1995 - PubMed
-
- Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN. MEF2C, a transcription factor that facilitates learning, and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci USA 105: 9391–9396, 2008 - PMC - PubMed
-
- Basel-Vanagaite L, Attia R, Yahav M, Ferland RJ, Anteki L, Walsh CA, Olender T, Straussberg R, Magal N, Taub E, Drasinover V, Alkelai A, Bercovich D, Rechavi G, Simon AJ, Shohat M. The CC2D1A, a member of a new gene family with C2 domains, is involved in autosomal recessive non-syndromic mental retardation. J Med Genet 43: 203–210, 2006 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

