Additive effects of Na+ and Cl- ions on barley growth under salinity stress
- PMID: 21273334
- PMCID: PMC3060698
- DOI: 10.1093/jxb/erq422
Additive effects of Na+ and Cl- ions on barley growth under salinity stress
Abstract
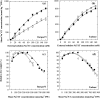
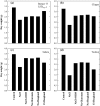
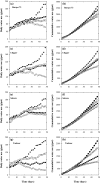
Soil salinity affects large areas of the world's cultivated land, causing significant reductions in crop yield. Despite the fact that most plants accumulate both sodium (Na(+)) and chloride (Cl(-)) ions in high concentrations in their shoot tissues when grown in saline soils, most research on salt tolerance in annual plants has focused on the toxic effects of Na(+) accumulation. It has previously been suggested that Cl(-) toxicity may also be an important cause of growth reduction in barley plants. Here, the extent to which specific ion toxicities of Na(+) and Cl(-) reduce the growth of barley grown in saline soils is shown under varying salinity treatments using four barley genotypes differing in their salt tolerance in solution and soil-based systems. High Na(+), Cl(-), and NaCl separately reduced the growth of barley, however, the reductions in growth and photosynthesis were greatest under NaCl stress and were mainly additive of the effects of Na(+) and Cl(-) stress. The results demonstrated that Na(+) and Cl(-) exclusion among barley genotypes are independent mechanisms and different genotypes expressed different combinations of the two mechanisms. High concentrations of Na(+) reduced K(+) and Ca(2+) uptake and reduced photosynthesis mainly by reducing stomatal conductance. By comparison, high Cl(-) concentration reduced photosynthetic capacity due to non-stomatal effects: there was chlorophyll degradation, and a reduction in the actual quantum yield of PSII electron transport which was associated with both photochemical quenching and the efficiency of excitation energy capture. The results also showed that there are fundamental differences in salinity responses between soil and solution culture, and that the importance of the different mechanisms of salt damage varies according to the system under which the plants were grown.
Figures
References
-
- Amtmann A, Sanders D. Mechanisms of Na uptake by plant cells. Advances in Botanical Research. 1998;29:75–112.
-
- Apse MP, Blumwald E. Na+ transport in plants. FEBS Letters. 2007;581:2247–2254. - PubMed
-
- Aydi S, Sassi S, Abdelly C. Growth, nitrogen fixation and ion distribution in Medicago truncatula subjected to salt stress. Plant and Soil. 2008;312:59–67.
-
- Ball MC, Chow WS, Anderson JM. Salinity-induced potassium deficiency causes loss of functional photosystem II in leaves of the grey mangrove, Avicennia marina, through depletion of the atrazine-binding polypeptide. Australian Journal of Plant Physiology. 1987;14:351–361.
-
- Boursier P, Läuchli A. Mechanisms of chloride partitioning in the leaves of salt-stressed Sorghum bicolor L. Physiologia Plantarum. 1989;77:537–544.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

