Antigen-specific splenic CD4+ and CD8+ regulatory T cells generated via the eye, suppress experimental autoimmune encephalomyelitis either at the priming or at the effector phase
- PMID: 21273399
- PMCID: PMC3030727
- DOI: 10.1093/intimm/dxq461
Antigen-specific splenic CD4+ and CD8+ regulatory T cells generated via the eye, suppress experimental autoimmune encephalomyelitis either at the priming or at the effector phase
Abstract
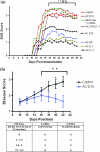
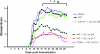
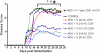
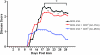
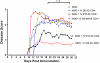
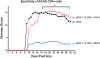
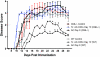
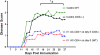
The injection of antigen into the ocular anterior chamber (AC) induces the generation of splenic CD4(+) and CD8(+) regulatory T (Treg) cells, specific for the antigen injected into the AC. These Treg cells inhibit the induction (CD4(+)) and also the expression (CD8(+)) of a delayed-type hypersensitivity response. The ability of AC-induced self-antigen-specific Treg cells in modulating autoimmunity is not well defined. Here we show that an injection of encephalitogenic myelin oligodendrocyte glycoprotein (MOG(35-55)) peptide into the anterior chamber of the eye (AC-MOG), before the induction of or during established experimental autoimmune encephalomyelitis (EAE) induced by MOG(35-55), suppresses the induction or progression of EAE, respectively. CD4(+) or CD8(+) splenic Treg cells induced by an injection of AC-MOG prevent EAE either at the inductive (priming) or at the progressive (effector) phase, respectively. This suppression of EAE by an AC-MOG injection or by intravenous transfer of splenic regulatory cells induced by an AC-MOG injection is specific for the antigen injected into the AC. Additionally, our data suggest that splenic CD8(+) Treg cells that suppress active EAE may use a transforming growth factor (TGF)-β-dependent suppression mechanism while the suppression of the induction of EAE by the AC-induced CD4(+) Treg cells is independent of TGF-β. Thus, we show for the first time that regulation of EAE at the priming or the chronic phase requires different phenotypes of Treg cells. Hence, it is important to consider the phenotype of Treg cells while designing effective cell-based therapies against autoimmune disorders.
Figures
References
-
- Niederkorn JY. Regulatory T cells and the eye. Chem. Immunol. Allergy. 2007;92:131. - PubMed
-
- Niederkorn JY. The induction of anterior chamber-associated immune deviation. Chem. Immunol. Allergy. 2007;92:27. - PubMed
-
- Niederkorn JY, Stein-Streilein J. History and physiology of immune privilege. Ocul. Immunol. Inflamm. 18:19. - PubMed
-
- Stein-Streilein J. Immune regulation and the eye. Trends. Immunol. 2008;29:548. - PubMed
-
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003;3:879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

