Theta-burst transcranial magnetic stimulation alters cortical inhibition
- PMID: 21273404
- PMCID: PMC6623597
- DOI: 10.1523/JNEUROSCI.1379-10.2011
Theta-burst transcranial magnetic stimulation alters cortical inhibition
Abstract
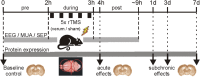
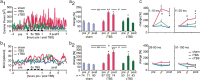
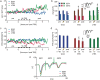
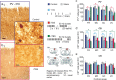
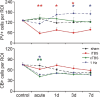
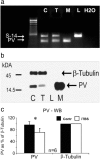
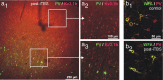
Human cortical excitability can be modified by repetitive transcranial magnetic stimulation (rTMS), but the cellular mechanisms are largely unknown. Here, we show that the pattern of delivery of theta-burst stimulation (TBS) (continuous versus intermittent) differently modifies electric activity and protein expression in the rat neocortex. Intermittent TBS (iTBS), but not continuous TBS (cTBS), enhanced spontaneous neuronal firing and EEG gamma band power. Sensory evoked cortical inhibition increased only after iTBS, although both TBS protocols increased the first sensory response arising from the resting cortical state. Changes in the cortical expression of the calcium-binding proteins parvalbumin (PV) and calbindin D-28k (CB) indicate that changes in spontaneous and evoked cortical activity following rTMS are in part related to altered activity of inhibitory systems. By reducing PV expression in the fast-spiking interneurons, iTBS primarily affected the inhibitory control of pyramidal cell output activity, while cTBS, by reducing CB expression, more likely affected the dendritic integration of synaptic inputs controlled by other classes of inhibitory interneurons. Calretinin, the third major calcium-binding protein expressed by another class of interneurons was not affected at all. We conclude that different patterns of TBS modulate the activity of inhibitory cell classes differently, probably depending on the synaptic connectivity and the preferred discharge pattern of these inhibitory neurons.
Figures

References
-
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. - PubMed
-
- Allison T. Recovery functions of somatosensory evoked responses in man. Electroencephalogr Clin Neurophysiol. 1962;14:331–343. - PubMed
-
- Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188:249–261. - PubMed
-
- Baumgärtel K, Tweedie-Cullen RY, Grossmann J, Gehrig P, Livingstone-Zatchej M, Mansuy IM. Changes in the proteome after neuronal zif268 overexpression. J Proteome Res. 2009;8:3298–3316. - PubMed
-
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
