The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity
- PMID: 21273411
- PMCID: PMC3078724
- DOI: 10.1523/JNEUROSCI.4545-10.2011
The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity
Abstract
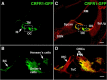
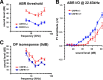
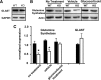
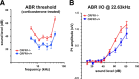
Cells of the inner ear face constant metabolic and structural stress. Exposure to intense sound or certain drugs destroys cochlea hair cells, which in mammals do not regenerate. Thus, an endogenous stress response system may exist within the cochlea to protect it from everyday stressors. We recently described the existence of corticotropin-releasing factor (CRF) in the mouse cochlea. The CRF receptor type 1 (CRFR1) is considered the primary and canonical target of CRF signaling, and systemically it plays an essential role in coordinating the body-wide stress response via activation of the hypothalamic-pituitary-adrenal (HPA) axis. Here, we describe an essential role for CRFR1 in auditory system development and function, and offer the first description of a complete HPA equivalent signaling system resident within the cochlea. To reveal the role of CRFR1 activation in the cochlea, we have used mice carrying a null ablation of the CRFR1 gene. CRFR1(-/-) mice exhibited elevated auditory thresholds at all frequencies tested, indicating reduced sensitivity. Furthermore, our results suggest that CRFR1 has a developmental role affecting inner hair cell morphology and afferent and efferent synapse distribution. Given the role of HPA signaling in maintaining local homeostasis in other tissues, the presence of a cochlear HPA signaling system suggests important roles for CRFR1 activity in setting cochlear sensitivity, perhaps both neural and non-neural mechanisms. These data highlight the complex pleiotropic mechanisms modulated by CRFR1 signaling in the cochlea.
Figures



Similar articles
-
The cochlear CRF signaling systems and their mechanisms of action in modulating cochlear sensitivity and protection against trauma.Mol Neurobiol. 2011 Dec;44(3):383-406. doi: 10.1007/s12035-011-8203-3. Epub 2011 Sep 11. Mol Neurobiol. 2011. PMID: 21909974 Free PMC article. Review.
-
Local Corticotropin-Releasing Factor Signaling in the Hypothalamic Paraventricular Nucleus.J Neurosci. 2018 Feb 21;38(8):1874-1890. doi: 10.1523/JNEUROSCI.1492-17.2017. Epub 2018 Jan 19. J Neurosci. 2018. PMID: 29352046 Free PMC article.
-
CRF-R1 activation in the anterior-dorsal BNST induces maternal neglect in lactating rats via an HPA axis-independent central mechanism.Psychoneuroendocrinology. 2016 Feb;64:89-98. doi: 10.1016/j.psyneuen.2015.11.015. Epub 2015 Nov 26. Psychoneuroendocrinology. 2016. PMID: 26630389 Free PMC article.
-
Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress.Nat Neurosci. 2017 Mar;20(3):385-388. doi: 10.1038/nn.4491. Epub 2017 Jan 30. Nat Neurosci. 2017. PMID: 28135239
-
Cellular signaling protective against noise-induced hearing loss – A role for novel intrinsic cochlear signaling involving corticotropin-releasing factor?Biochem Pharmacol. 2015 Sep 1;97(1):1-15. doi: 10.1016/j.bcp.2015.06.011. Epub 2015 Jun 11. Biochem Pharmacol. 2015. PMID: 26074267 Free PMC article. Review.
Cited by
-
The cochlear CRF signaling systems and their mechanisms of action in modulating cochlear sensitivity and protection against trauma.Mol Neurobiol. 2011 Dec;44(3):383-406. doi: 10.1007/s12035-011-8203-3. Epub 2011 Sep 11. Mol Neurobiol. 2011. PMID: 21909974 Free PMC article. Review.
-
Stress and tinnitus-from bedside to bench and back.Front Syst Neurosci. 2012 Jun 11;6:47. doi: 10.3389/fnsys.2012.00047. eCollection 2012. Front Syst Neurosci. 2012. PMID: 22701404 Free PMC article.
-
Spatial Gradients in the Size of Inner Hair Cell Ribbons Emerge Before the Onset of Hearing in Rats.J Assoc Res Otolaryngol. 2017 Jun;18(3):399-413. doi: 10.1007/s10162-017-0620-1. Epub 2017 Mar 30. J Assoc Res Otolaryngol. 2017. PMID: 28361374 Free PMC article.
-
Contralateral-noise effects on cochlear responses in anesthetized mice are dominated by feedback from an unknown pathway.J Neurophysiol. 2012 Jul;108(2):491-500. doi: 10.1152/jn.01050.2011. Epub 2012 Apr 18. J Neurophysiol. 2012. PMID: 22514298 Free PMC article.
-
Partial requirement of endothelin receptor B in spiral ganglion neurons for postnatal development of hearing.J Biol Chem. 2011 Aug 26;286(34):29621-6. doi: 10.1074/jbc.M111.236802. Epub 2011 Jun 28. J Biol Chem. 2011. PMID: 21715336 Free PMC article.
References
-
- Brunton PJ, Sausbier M, Wietzorrek G, Sausbier U, Knaus HG, Russell JA, Ruth P, Shipston MJ. Hypothalamic-pituitary-adrenal axis hyporesponsiveness to restraint stress in mice deficient for large-conductance calcium- and voltage-activated potassium (BK) channels. Endocrinology. 2007;148:5496–5506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
