Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior
- PMID: 21273413
- PMCID: PMC6623632
- DOI: 10.1523/JNEUROSCI.3779-10.2011
Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior
Abstract
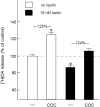
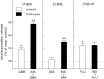
Because insulin acutely enhances the function of dopamine transporters, the tyrosine kinase receptors activated by this hormone may modulate transporter-dependent neurochemical and behavioral effects of psychoactive drugs. In this respect, we examined the effects of insulin on exocytotic monoamine release and the efficacy of the monoamine transporter blocker cocaine in rat nucleus accumbens. Whereas insulin reduced electrically evoked exocytotic [(3)H]dopamine release in nucleus accumbens slices, the hormone potentiated the release-enhancing effect of cocaine thereon. The phosphatidylinositol 3-kinase inhibitor LY294002 abolished these effects, indicating the involvement of insulin receptors. Similar insulin effects were observed on the release of [(3)H]norepinephrine in nucleus accumbens slices, but not on that of [(3)H]serotonin, and were also apparent in medial prefrontal cortex slices. As might then be expected, insulin also potentiated the dopamine and norepinephrine release-enhancing effects of the selective monoamine uptake inhibitors GBR12909 and desmethylimipramine, respectively. In subsequent behavioral experiments, we investigated the role of insulin in motor impulsivity that depends on monoamine neurotransmission in the nucleus accumbens. Intracranial administration of insulin in the nucleus accumbens alone reduced premature responses in the five-choice serial reaction time task and enhanced the stimulatory effect of peripheral cocaine administration on impulsivity, resembling the observed neurochemical effects of the hormone. In contrast, cocaine-induced locomotor activity remained unchanged by intra-accumbal insulin application. These data reveal that insulin presynaptically regulates cocaine-sensitive monoamine transporter function in the nucleus accumbens and, as a consequence, impulsivity. Therefore, insulin signaling proteins may represent targets for the treatment of inhibitory control deficits such as addictive behaviors.
Figures





Similar articles
-
Cocaine acts on accumbens monoamines and locomotor behavior via a 5-HT2A/2C receptor mechanism as shown by ketanserin: 24-h follow-up studies.Prog Neuropsychopharmacol Biol Psychiatry. 2004 May;28(3):547-57. doi: 10.1016/j.pnpbp.2004.01.007. Prog Neuropsychopharmacol Biol Psychiatry. 2004. PMID: 15093963
-
Clozapine and cocaine effects on dopamine and serotonin release in nucleus accumbens during psychostimulant behavior and withdrawal.Prog Neuropsychopharmacol Biol Psychiatry. 2004 Jan;28(1):157-71. doi: 10.1016/j.pnpbp.2003.09.032. Prog Neuropsychopharmacol Biol Psychiatry. 2004. PMID: 14687870
-
Extracellular dopamine, norepinephrine, and serotonin in the nucleus accumbens of freely moving rats during intracerebral dialysis with cocaine and other monoamine uptake blockers.J Neurochem. 1996 Feb;66(2):559-68. doi: 10.1046/j.1471-4159.1996.66020559.x. J Neurochem. 1996. PMID: 8592125
-
Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms.Eur J Pharmacol. 2015 Oct 5;764:562-570. doi: 10.1016/j.ejphar.2015.07.044. Epub 2015 Jul 21. Eur J Pharmacol. 2015. PMID: 26209364 Free PMC article. Review.
-
Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions.Ann N Y Acad Sci. 2001 Jun;937:93-120. doi: 10.1111/j.1749-6632.2001.tb03560.x. Ann N Y Acad Sci. 2001. PMID: 11458542 Review.
Cited by
-
Intranasal Insulin: a Treatment Strategy for Addiction.Neurotherapeutics. 2020 Jan;17(1):105-115. doi: 10.1007/s13311-019-00822-4. Neurotherapeutics. 2020. PMID: 31898283 Free PMC article. Review.
-
Importance of cholesterol in dopamine transporter function.J Neurochem. 2012 Dec;123(5):700-15. doi: 10.1111/jnc.12007. Epub 2012 Oct 11. J Neurochem. 2012. PMID: 22957537 Free PMC article.
-
Reduced insulin sensitivity is related to less endogenous dopamine at D2/3 receptors in the ventral striatum of healthy nonobese humans.Int J Neuropsychopharmacol. 2015 Feb 25;18(7):pyv014. doi: 10.1093/ijnp/pyv014. Int J Neuropsychopharmacol. 2015. PMID: 25716779 Free PMC article.
-
Dendritic Release of Neurotransmitters.Compr Physiol. 2016 Dec 6;7(1):235-252. doi: 10.1002/cphy.c160007. Compr Physiol. 2016. PMID: 28135005 Free PMC article. Review.
-
Impulsive Personality Traits Predicted Weight Loss in Individuals with Type 2 Diabetes after 3 Years of Lifestyle Interventions.J Clin Med. 2022 Jun 16;11(12):3476. doi: 10.3390/jcm11123476. J Clin Med. 2022. PMID: 35743546 Free PMC article.
References
-
- Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986–2992. - PubMed
-
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. - PubMed
-
- Carvelli L, Morón JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. - PubMed
-
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
