Multiple targets of μ-opioid receptor-mediated presynaptic inhibition at primary afferent Aδ- and C-fibers
- PMID: 21273416
- PMCID: PMC6623607
- DOI: 10.1523/JNEUROSCI.4060-10.2011
Multiple targets of μ-opioid receptor-mediated presynaptic inhibition at primary afferent Aδ- and C-fibers
Abstract
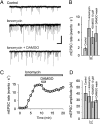
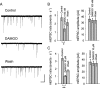
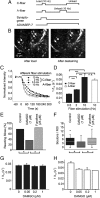
Agonists at μ-opioid receptors (MORs) represent the gold standard for the treatment of severe pain. A key element of opioid analgesia is the depression of nociceptive information at the first synaptic relay in spinal pain pathways. The underlying mechanisms are, however, largely unknown. In spinal cord slices with dorsal roots attached prepared from young rats, we determined the inhibitory effect of the selective MOR agonist [d-Ala(2), N-Me-Phe(4), Gly(5)-ol]-enkephalin (DAMGO) on monosynaptic Aδ- and C-fiber-evoked EPSCs in lamina I neurons. DAMGO depressed presynaptically Aδ- and C-fiber-mediated responses, indicating that MORs are expressed on central terminals of both fiber types. We next addressed the mechanisms of presynaptic inhibition. The effect of DAMGO at both Aδ- and C-fiber terminals was mainly mediated by an inhibition of N-type voltage-dependent Ca(2+) channels (VDCCs), and to a lesser extent of P/Q-type VDCCs. Inhibition by DAMGO was not reduced by K(+) channel blockers. The rate of miniature EPSCs was reduced by DAMGO in a dose-dependent manner. The opioid also reduced Ca(2+)-dependent, ionomycin-induced EPSCs downstream of VDCCs. DAMGO had no effect on the kinetics of vesicle exocytosis in C-fiber terminals, but decreased the rate of unloading of Aδ-fiber boutons moderately, as revealed by two-photon imaging of styryl dye destaining. Together, these results suggest that binding of opioids to MORs reduces nociceptive signal transmission at central Aδ- and C-fiber synapses mainly by inhibition of presynaptic N-type VDCCs. P/Q-type VDCCs and the transmitter release machinery are targets of opioid action as well.
Figures



Similar articles
-
Differential presynaptic effects of opioid agonists on Adelta- and C-afferent glutamatergic transmission to the spinal dorsal horn.Anesthesiology. 2007 Nov;107(5):807-12. doi: 10.1097/01.anes.0000286985.80301.5e. Anesthesiology. 2007. PMID: 18073556
-
Sustained inhibition of neurotransmitter release from nontransient receptor potential vanilloid type 1-expressing primary afferents by mu-opioid receptor activation-enkephalin in the spinal cord.J Pharmacol Exp Ther. 2008 Nov;327(2):375-82. doi: 10.1124/jpet.108.141226. Epub 2008 Jul 31. J Pharmacol Exp Ther. 2008. PMID: 18669865
-
Opioid-induced long-term potentiation in the spinal cord is a presynaptic event.J Neurosci. 2010 Mar 24;30(12):4460-6. doi: 10.1523/JNEUROSCI.5857-09.2010. J Neurosci. 2010. PMID: 20335482 Free PMC article.
-
mu-Opioid receptor inhibits N-type Ca2+ channels in the calyx presynaptic terminal of the embryonic chick ciliary ganglion.J Physiol. 2000 May 1;524 Pt 3(Pt 3):769-81. doi: 10.1111/j.1469-7793.2000.00769.x. J Physiol. 2000. PMID: 10790157 Free PMC article.
-
Mu opiates inhibit long-term potentiation induction in the spinal cord slice.J Neurophysiol. 2001 Feb;85(2):485-94. doi: 10.1152/jn.2001.85.2.485. J Neurophysiol. 2001. PMID: 11160487
Cited by
-
Morphine Efficacy, Tolerance, and Hypersensitivity Are Altered After Modulation of SUR1 Subtype KATP Channel Activity in Mice.Front Neurosci. 2019 Oct 22;13:1122. doi: 10.3389/fnins.2019.01122. eCollection 2019. Front Neurosci. 2019. PMID: 31695594 Free PMC article.
-
Phorbol ester modulation of Ca2+ channels mediates nociceptive transmission in dorsal horn neurones.Pharmaceuticals (Basel). 2013 May 29;6(6):777-87. doi: 10.3390/ph6060777. Pharmaceuticals (Basel). 2013. PMID: 24276261 Free PMC article.
-
Kir3 channel signaling complexes: focus on opioid receptor signaling.Front Cell Neurosci. 2014 Jul 8;8:186. doi: 10.3389/fncel.2014.00186. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25071446 Free PMC article. Review.
-
G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury.Mol Pain. 2016 Dec 7;12:1744806916682242. doi: 10.1177/1744806916682242. Print 2016. Mol Pain. 2016. PMID: 27927796 Free PMC article.
-
Functions of Presynaptic Voltage-gated Calcium Channels.Function (Oxf). 2021;2(1):zqaa027. doi: 10.1093/function/zqaa027. Epub 2020 Oct 23. Function (Oxf). 2021. PMID: 33313507 Free PMC article. Review.
References
-
- Abbadie C, Pasternak GW, Aicher SA. Presynaptic localization of the carboxy-terminus epitopes of the μ opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience. 2001;106:833–842. - PubMed
-
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. - PubMed
-
- Barral J, Mendoza E, Galarraga E, Bargas J. The presynaptic modulation of corticostriatal afferents by μ-opioids is mediated by K+ conductances. Eur J Pharmacol. 2003;462:91–98. - PubMed
-
- Bergevin A, Girardot D, Bourque M-J, Trudeau L-E. Presynaptic μ-opioid receptors regulate a late step of the secretory process in rat ventral tegmental area GABAergic neurons. Neuropharmacology. 2002;42:1065–1078. - PubMed
-
- Besse D, Lombard M-C, Zajac J-M, Roques BP, Besson J-M. Pre- and postsynaptic distribution of μ, δ and κ opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
