The role of striatal tonically active neurons in reward prediction error signaling during instrumental task performance
- PMID: 21273435
- PMCID: PMC6623604
- DOI: 10.1523/JNEUROSCI.4880-10.2011
The role of striatal tonically active neurons in reward prediction error signaling during instrumental task performance
Abstract
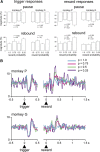
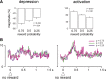
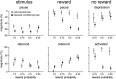
The detection of differences between predictions and actual outcomes is important for associative learning and for selecting actions according to their potential future reward. There are reports that tonically active neurons (TANs) in the primate striatum may carry information about errors in the prediction of rewards. However, this property seems to be expressed in classical conditioning tasks but not during performance of an instrumental task. To address this issue, we recorded the activity of TANs in the putamen of two monkeys performing an instrumental task in which probabilistic rewarding outcomes were contingent on an action in block-design experiments. Behavioral evidence suggests that animals adjusted their performance according to the level of probability for reward on each trial block. We found that the TAN response to reward was stronger as the reward probability decreased; this effect was especially prominent on the late component of the pause-rebound pattern of typical response seen in these neurons. The responsiveness to reward omission was also increased with increasing reward probability, whereas there were no detectable effects on responses to the stimulus that triggered the movement. Overall, the modulation of TAN responses by reward probability appeared relatively weak compared with that observed previously in a probabilistic classical conditioning task using the same block design. These data indicate that instrumental conditioning was less effective at demonstrating prediction error signaling in TANs. We conclude that the sensitivity of the TAN system to reward probability depends on the specific learning situation in which animals experienced the stimulus-reward associations.
Figures



References
-
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol. 1995;73:1234–1252. - PubMed
-
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. - PubMed
-
- Apicella P, Legallet E, Trouche E. Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Exp Brain Res. 1997;116:456–466. - PubMed
-
- Apicella P, Deffains M, Ravel S, Legallet E. Tonically active neurons in the striatum differentiate between delivery and omission of expected reward in a probabilistic task context. Eur J Neurosci. 2009;30:515–526. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
