ROS signaling by NOX4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma
- PMID: 21273445
- PMCID: PMC5417274
- DOI: 10.1210/me.2010-0340
ROS signaling by NOX4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma
Abstract
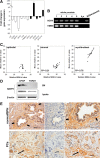
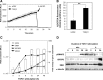
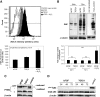
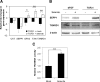
Stromal remodeling, in particular fibroblast-to-myofibroblast differentiation, is a hallmark of benign prostatic hyperplasia (BPH) and solid tumors, including prostate cancer (PCa). Increased local production of TGFβ1 is considered the inducing stimulus. Given that stromal remodeling actively promotes BPH/PCa development, there is considerable interest in developing stromal-targeted therapies. Microarray and quantitative PCR analysis of primary human prostatic stromal cells induced to undergo fibroblast-to-myofibroblast differentiation with TGFβ1 revealed up-regulation of the reactive oxygen species (ROS) producer reduced nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) and down-regulation of the selenium-containing ROS-scavenging enzymes glutathione peroxidase 3, thioredoxin reductase 1 (TXNRD1), and the selenium transporter selenoprotein P plasma 1. Consistently, NOX4 expression correlated specifically with the myofibroblast phenotype in vivo, and loss of selenoprotein P plasma 1 was observed in tumor-associated stroma of human PCa biopsies. Using lentiviral NOX4 short hairpin RNA-mediated knockdown, pharmacological inhibitors, antioxidants, and selenium, we demonstrate that TGFβ1 induction of NOX4-derived ROS is required for TGFβ1-mediated phosphorylation of c-jun N-terminal kinase, which in turn is essential for subsequent downstream cytoskeletal remodeling. Significantly, selenium supplementation inhibited differentiation by increasing ROS-scavenging selenoenzyme biosynthesis because glutathione peroxidase 3 and TXNRD1 expression and TXNRD1 enzyme activity were restored. Consistently, selenium depleted ROS levels downstream of NOX4 induction. Collectively, this work demonstrates that dysregulated redox homeostasis driven by elevated NOX4-derived ROS signaling underlies fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Further, these data indicate the potential clinical value of selenium and/or NOX4 inhibitors in preventing the functional pathogenic changes of stromal cells in BPH and PCa.
Figures


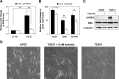
References
-
- Sampson N , Untergasser G , Plas E , Berger P. 2007. The ageing male reproductive tract. J Pathol 211:206–218 - PubMed
-
- Isaacs JT. 1994. Etiology of benign prostatic hyperplasia. Eur Urol 25(Suppl 1):6–9 - PubMed
-
- Jemal A , Siegel R , Ward E , Murray T , Xu J , Smigal C , Thun MJ. 2006. Cancer statistics, 2006. CA Cancer J Clin 56:106–130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

