Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome
- PMID: 21273506
- PMCID: PMC3041065
- DOI: 10.1073/pnas.1018823108
Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome
Abstract
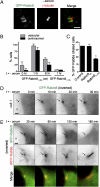
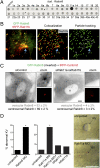
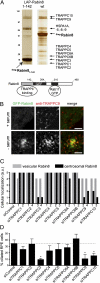
Sensory and signaling pathways are exquisitely organized in primary cilia. Bardet-Biedl syndrome (BBS) patients have compromised cilia and signaling. BBS proteins form the BBSome, which binds Rabin8, a guanine nucleotide exchange factor (GEF) activating the Rab8 GTPase, required for ciliary assembly. We now describe serum-regulated upstream vesicular transport events leading to centrosomal Rab8 activation and ciliary membrane formation. Using live microscopy imaging, we show that upon serum withdrawal Rab8 is observed to assemble the ciliary membrane in ∼100 min. Rab8-dependent ciliary assembly is initiated by the relocalization of Rabin8 to Rab11-positive vesicles that are transported to the centrosome. After ciliogenesis, Rab8 ciliary transport is strongly reduced, and this reduction appears to be associated with decreased Rabin8 centrosomal accumulation. Rab11-GTP associates with the Rabin8 COOH-terminal region and is required for Rabin8 preciliary membrane trafficking to the centrosome and for ciliogenesis. Using zebrafish as a model organism, we show that Rabin8 and Rab11 are associated with the BBS pathway. Finally, using tandem affinity purification and mass spectrometry, we determined that the transport protein particle (TRAPP) II complex associates with the Rabin8 NH(2)-terminal domain and show that TRAPP II subunits colocalize with centrosomal Rabin8 and are required for Rabin8 preciliary targeting and ciliogenesis.
Conflict of interest statement
Conflict of interest statement: C.J.W., K.J.W., K.E.E., L.P., C.C., D.S.K., R.H.S., and P.K.J. are employees of Genentech, Inc.
Figures

References
-
- Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. - PubMed
-
- Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. - PubMed
-
- Yen HJ, et al. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet. 2006;15:667–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

