Accurate classification of metastatic brain tumors using a novel microRNA-based test
- PMID: 21273512
- PMCID: PMC3228095
- DOI: 10.1634/theoncologist.2010-0305
Accurate classification of metastatic brain tumors using a novel microRNA-based test
Abstract
Background: Identification of the tissue of origin of a brain metastatic tumor is vital to its management. Carcinoma of unknown primary (CUP) is common in oncology, representing 3%-5% of all invasive malignancies. We aimed to validate a recently developed microRNA-based quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) test for identifying the tumor tissue of origin, first in a consecutive cohort of metastatic tumors of known origin and then in a cohort of CUP cases resected from the central nervous system (CNS).
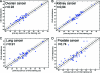
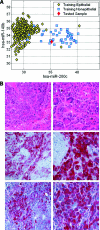
Patients and methods: One hundred two resected CNS metastatic tumors with known origin, previously classified based on the patient's clinical history and pathological data, as well as a second cohort of resected CNS tumors from 57 patients originally diagnosed as CUP were studied. A qRT-PCR diagnostic assay that measures the expression level of 48 microRNAs was used to classify the tissue of origin of these metastatic tumors.
Results: In this blinded study, the test predictions correctly identified the reference diagnosis of the samples of known origin, excluding samples from prostate origin, in 84% of cases. In the second CUP patient cohort, the test prediction was in agreement with the diagnosis that was later confirmed clinically or with pathological evaluation in 80% of cases.
Conclusion: In a cohort of brain and spinal metastases, a previously developed test based on the expression of 48 microRNAs allowed accurate identification of the tumor tissue of origin in the majority of cases. The high accuracy of this test in identifying the tissue of origin of metastases of unknown primary is demonstrated for the first time and may have broad clinical application.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures

References
-
- Suki D. The epidemiology of brain metastases. In: Sawaya R, editor. Intracranial Metastases: Current Management Strategies. Malden, MA: Blackwell Futura Publishing; 2004. pp. 20–34.
-
- Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. - PubMed
-
- Becher MW, Abel TW, Thompson RC, et al. Immunohistochemical analysis of metastatic neoplasms of the central nervous system. J Neuropathol Exp Neurol. 2006;65:935–944. - PubMed
-
- Drlicek M, Bodenteich A, Urbanits S, et al. Immunohistochemical panel of antibodies in the diagnosis of brain metastases of the unknown primary. Pathol Res Pract. 2004;200:727–734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

