Principles of and advances in percutaneous ablation
- PMID: 21273519
- PMCID: PMC6939957
- DOI: 10.1148/radiol.10081634
Principles of and advances in percutaneous ablation
Abstract
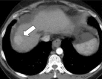
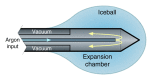
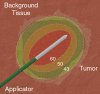
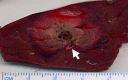
Image-guided tumor ablation with both thermal and nonthermal sources has received substantial attention for the treatment of many focal malignancies. Increasing interest has been accompanied by continual advances in energy delivery, application technique, and therapeutic combinations with the intent to improve the efficacy and/or specificity of ablative therapies. This review outlines clinical percutaneous tumor ablation technology, detailing the science, devices, techniques, technical obstacles, current trends, and future goals in percutaneous tumor ablation. Methods such as chemical ablation, cryoablation, high-temperature ablation (radiofrequency, microwave, laser, and ultrasound), and irreversible electroporation will be discussed. Advances in technique will also be covered, including combination therapies, tissue property modulation, and the role of computer modeling for treatment optimization.
© RSNA, 2011.
Figures


















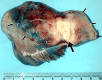




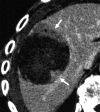





References
-
- Solbiati L, Ierace T, Cova L, Zaid S, Imparato S. Ten-year experience in nonsurgical treatment of “intermediate” hepatocellular carcinoma in cirrhotic patients: long-term survival and causes of death [abstr]. In: Radiological Society of North America scientific assembly and annual meeting program. Oak Brook, Ill: Radiological Society of North America, 2006;359.
-
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226(2):441–451. - PubMed
-
- Gillams AR, Lees WR. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol 2008;19(5):712–717. - PubMed
-
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 2003;226(2):417–424. - PubMed
-
- Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 2006;129(3):738–745. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

