Smad4 is required predominantly in the developmental processes dependent on the BMP branch of the TGF-β signaling system in the embryonic mouse retina
- PMID: 21273545
- PMCID: PMC3109008
- DOI: 10.1167/iovs.10-5940
Smad4 is required predominantly in the developmental processes dependent on the BMP branch of the TGF-β signaling system in the embryonic mouse retina
Abstract
Purpose: The present study was aimed at defining developmental roles of Smad4, a key mediator of the TGF-β superfamily signaling system, in the embryonic mouse retina.
Methods: Using a Cre/loxP-mediated conditional gene targeting approach, Smad4 gene function was deleted from the embryonic mouse retina. Mutant phenotypes were morphologically and molecularly examined.
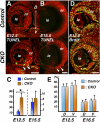
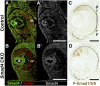
Results: Loss of Smad4 in the developing retina led to varying degrees of microphthalmia at birth, presumably because of elevated apoptosis observed transiently at embryonic day 12.5 in the developing retina. This was also associated with an apparent delay in accumulation of retinal ganglion cells. Smad4 conditional mutants also exhibited alterations of retinal spatial patterning along the dorsal-ventral axis, consistent with a known function of BMP signaling in the embryonic retina. However, despite a known role for BMP signaling in retinal cell survival, proliferation, and differentiation, Smad4 mutant retinal progenitor cells were capable of maintaining growth and neurogenesis throughout embryonic development. We also found that the loss of Smad4 led to abnormal targeting of retinal ganglion cell axons to the optic nerve head, a phenotype consistent with reduced BMP signaling in the developing retina.
Conclusions: These results suggest that Smad4 is essential for a subset of, but not all, TGF-β/BMP-dependent developmental processes in the embryonic retina. In addition, genetic requirements for Smad4 in the embryonic retina are evident predominantly in the developmental events regulated by the BMP branch of the TGF-β signaling pathway.
Figures




References
-
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21:367–378 - PubMed
-
- Duenker N. Transforming growth factor-beta (TGF-beta) and programmed cell death in the vertebrate retina. Int Rev Cytol. 2005;245:17–43 - PubMed
-
- Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood). 2007;232:979–992 - PubMed
-
- Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci. 2003;8:d855–869 - PubMed
-
- Adler R, Belecky-Adams TL. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development. 2002;129:3161–3171 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

